B21, China Town Mall, Midrand
Vacuum Therapy Breast Enhancement Nipple Stretching Massager:
- Section : Beauty & Personal Care
- Category : Beauty Equipment
- SKU : 62189455747

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 12 Mar, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
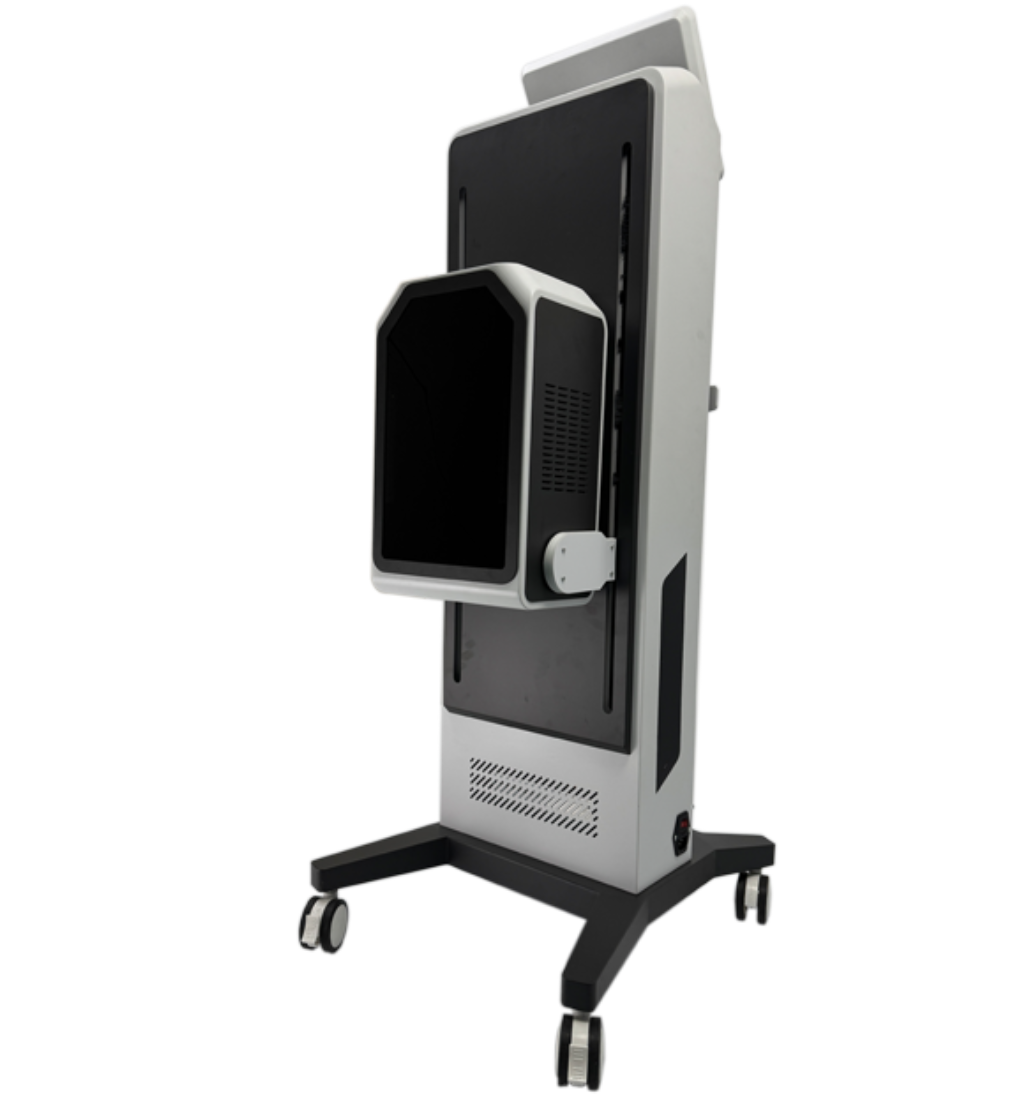
1. What exactly is the product — a vacuum breast therapy massager or the GLM H-031II facial treatment device?
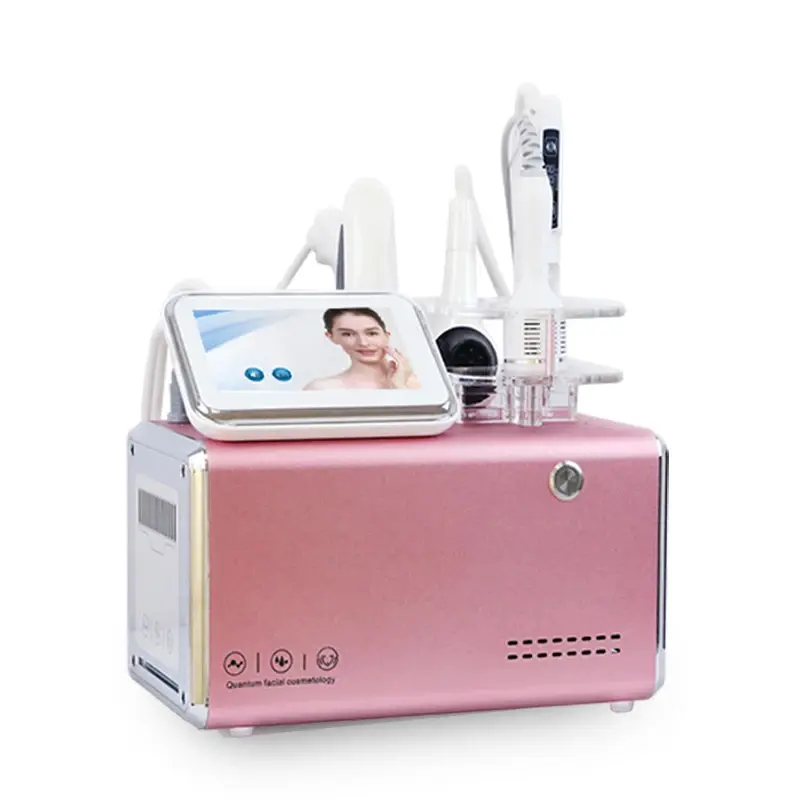
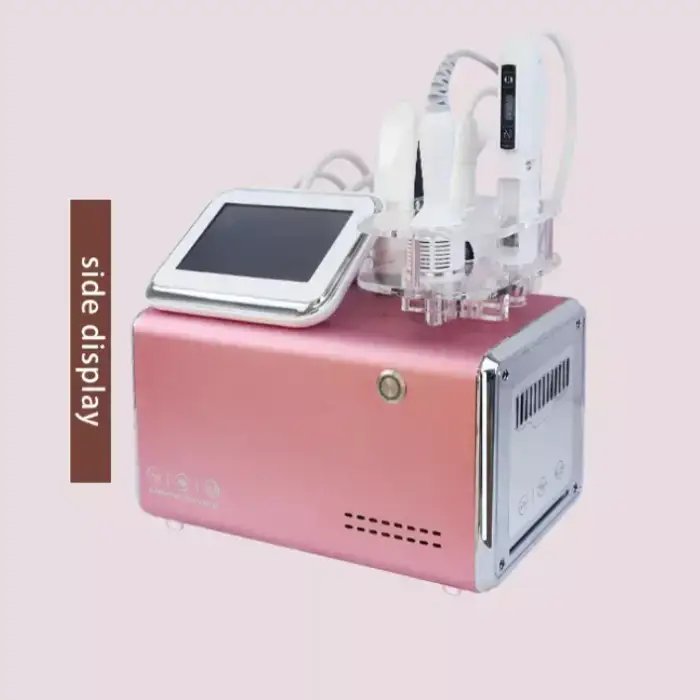
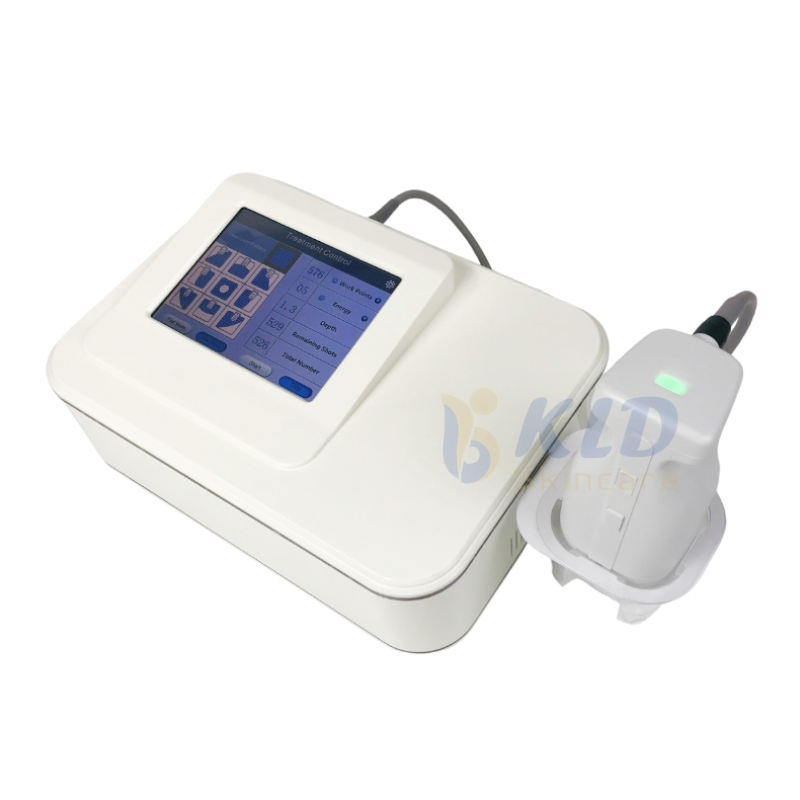
There appears to be a mismatch between the product name and the description. The name refers to a vacuum therapy breast enhancement / nipple stretching massager, while the description details the GLM H-031II Facial Treatment Face Lift Equipment (galvanic, RF, thermo, EMS, cooling). Confirm the SKU or product model with the seller to ensure you receive the correct device.
2. How does vacuum therapy for breast enhancement work?
Vacuum therapy uses controlled negative pressure (suction) to increase blood flow, promote lymphatic drainage, and temporarily expand soft tissue. It can produce short-term fullness and improved circulation, but results and longevity vary and professional protocols should be followed.
3. Is the GLM H-031II designed for breast enhancement or nipple stretching?
No. The GLM H-031II description outlines a facial treatment device (galvanic, RF, thermo facelift, EMS, cooling). It is not intended or certified for breast enhancement or nipple stretching. Do not use facial RF/EMS probes on breast tissue or nipples.
4. What technologies are included in the GLM H-031II facial machine?
The GLM H-031II incorporates galvanic technology, thermo facelift (controlled heat), radio frequency (RF), cooling therapy, electro muscle stimulation (EMS), bionic clip technology, and multiple adjustable air output modes for customized treatments.
5. Who should operate this equipment?
This device is intended for trained professionals — licensed aestheticians, dermatologists, or clinic staff with hands‑on training. It is not recommended for unsupervised home use unless the manufacturer offers a user version and training.
6. What are common contraindications and safety warnings?
Do not use on clients who are pregnant or breastfeeding (for breast devices), have pacemakers or implanted electronic devices, active cancer, open wounds, active infections, severe cardiovascular disease, or epilepsy. Avoid use over metal implants without medical clearance. Always follow the manufacturer safety list and obtain a medical history prior to treatment.
7. What side effects or risks can occur?
Common temporary effects include redness, mild swelling, bruising, tenderness, numbness, or tingling. Improper use can cause skin damage, burns (with RF/thermo), or bruising (with vacuum). Stop treatment and seek medical advice if severe pain, persistent bruising, blistering, or signs of infection occur.
8. How long is a typical facial treatment session with the GLM H-031II and how many sessions are needed?
Professional facial sessions typically last 20–40 minutes depending on the protocol and area treated. A course of 6–12 sessions spaced weekly or biweekly is common for cumulative collagen stimulation; individual recommendations should follow manufacturer protocols and a professional assessment.
9. How often can vacuum breast therapy be performed and when are results noticeable?
If using a dedicated vacuum breast device under professional supervision, sessions often last 15–30 minutes and may be done several times per week initially. Some immediate temporary fullness may be seen after treatment; sustained change requires repeated treatments and maintenance. Follow a professional plan — do not self-treat without guidance.
10. Can this device be used on all body areas?
The GLM H-031II is described for the face, neck, eye area and some body applications when appropriate handles are provided. Devices specifically named for breast or nipple treatment are configured with specialized suction cups and safety parameters — do not interchange probes between face and breast treatments unless explicitly approved by the manufacturer.
11. How should I clean and maintain the machine?
Follow the manufacturer cleaning instructions. General care: power off and unplug before cleaning, wipe external surfaces and probes with approved disinfectant, avoid immersing the unit in water despite ABS housing, inspect hoses and consumables for wear, replace disposable parts (filters/suction cups) as recommended, and store in a dry, cool place.
12. Are there accessories or consumables I need to buy separately?
Common accessories include conductive gels, disposable suction cups or cushions, replacement hoses, filters, electrode pads, and protective covers. Check the product listing or supplier for the included items and recommended consumables.
13. Is the device safe to use if a client has breast implants or recent surgery?
No treatment involving suction, RF, or strong mechanical manipulation should be used over recent surgical sites or implants without explicit medical clearance from the client’s surgeon. Consult a medical professional before treating clients with implants or recent procedures.
14. What training or certification is required to use this machine safely and effectively?
Operators should complete manufacturer-provided training and have appropriate professional licensure (aesthetician, cosmetologist, nurse, or physician depending on local regulations). Training covers device operation, contraindications, treatment protocols, client assessment, and emergency procedures.
15. What warranty and technical support should I expect?
Warranty terms vary by manufacturer and seller; commercial aesthetic devices commonly include a limited parts warranty (often 6–12 months) with optional extended plans. Confirm warranty length, what it covers (parts vs. consumables), return policy, and availability of technical support or replacement parts before purchase.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading