B21, China Town Mall, Midrand
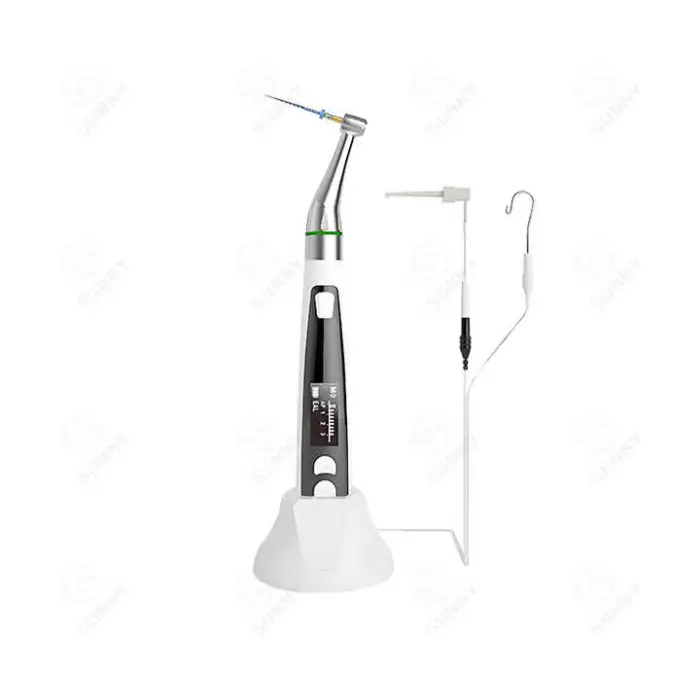
Wireless Endodontic Handpiece with Apex Locator Dental Electric Motor for Oral Therapy Equipments & Accessories
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1601442366253

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 03 Feb, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Wireless Endodontic Handpiece with Apex Locator?
It is a cordless electric endomotor designed for endodontic (root canal) treatment that integrates an apex locator for measuring working length, offering portable, precision oral therapy for dental professionals.
2. What are the key certifications and classifications of this device?
The device is classified as a Class II medical instrument and carries CE quality certification, indicating conformity with applicable European safety and performance standards.
3. How does the "wireless" feature work — does it require electricity?
The unit is an electric motor with wireless/portable operation, meaning it runs on internal power (rechargeable) rather than a constant external cable. For exact battery type, charging method and electrical specifications, consult the product manual or supplier.
4. What is the built-in apex locator and how accurate is it?
The built-in apex locator provides electronic measurements to help determine working length during root canal procedures. Accuracy depends on proper use and clinical technique; follow the manufacturer’s instructions and verify measurements as part of your normal clinical checks.
5. Which procedures is this handpiece intended for?
It is intended for endodontic treatments such as root canal preparation, and can be used in clinical practice, dental education, and training settings where precise length measurement and rotary instrumentation are required.
6. Is the handpiece compatible with standard endodontic rotary files and attachments?
The device is designed for typical endodontic use, but compatibility can vary by file shank and contra-angle type. Confirm compatibility with your specific rotary files and attachments with the supplier or product documentation before use.
7. How should I clean and sterilize the handpiece?
Clean and disinfect external surfaces according to the manufacturer’s instructions. Only detachable parts specifically marked as autoclavable should be sterilized; do not autoclave the entire motor unit unless explicitly permitted. Refer to the user manual for detailed cleaning and sterilization procedures.
8. What routine maintenance is required?
The product features service‑free spare parts for simplified upkeep, but routine tasks typically include keeping the unit clean, charging the battery as recommended, inspecting for wear or damage, and following any calibration/check procedures in the manual.
9. What is the shelf life and packaging information?
Shelf life is listed as 5 years. The single package dimensions are 30 x 20 x 17 cm, and the gross weight for a single packaged item is approximately 4.0 kg.
10. Is training required to use this device?
Yes. This is a professional dental instrument intended for use by trained dental clinicians or students under supervision. Proper training in endodontic technique and device operation is required to ensure safe and effective use.
11. Does the product come with a warranty or after-sales support?
Warranty and support details are not listed in the product description. Check with the seller or manufacturer for specific warranty terms, service options and authorized service centers.
12. Can the apex locator be calibrated or verified?
The apex locator should be used and checked according to the manufacturer’s recommendations. Some devices include self-check or calibration procedures; consult the user manual for verification, calibration steps and recommended quality-control checks.
13. Is the device safe to use on all patients?
As a Class II medical device with CE certification, it meets safety standards, but clinicians should assess each patient individually. Follow contraindications, infection-control protocols, and manufacturer guidance when treating patients with implants, pacemakers, or other special medical conditions.
14. What should I do if the handpiece stops charging or the apex locator is unresponsive?
First consult the troubleshooting section of the user manual for basic checks (connections, charging indicators, battery status). If the problem persists, contact the supplier or authorized service center for inspection and repair; do not attempt internal repairs unless authorized.
15. Where is this device best used — clinic, hospital or education?
It is suitable for dental clinics and hospitals as well as dental schools and training labs. Its portable wireless design also makes it convenient for mobile dentistry or outreach programs, provided infection-control and safety requirements are met.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals