B21, China Town Mall, Midrand
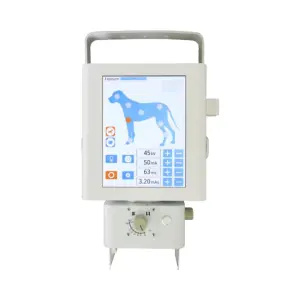
TJ-HE001 Intelligent Arm Hand Rehabilitation Device Class I Physical Therapy Equipment for Stroke Hemiplegia Recovery
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1601155896607

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 23 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the TJ-HE001 Intelligent Arm Hand Rehabilitation Device used for?
The TJ-HE001 is designed to assist individuals recovering from stroke and hemiplegia by supporting arm and hand rehabilitation exercises to help regain strength and mobility. It is intended for use in hospitals, rehabilitation centers and home care settings.
2. Who is an appropriate candidate to use this device?
The device is intended for people undergoing recovery from stroke-related hemiplegia or other upper-limb motor impairments. Use should be guided by a physician or licensed rehabilitation therapist to determine suitability for each patient.
3. Is the TJ-HE001 suitable for home use?
Yes. The device is described as lightweight and portable and is suitable for home use as well as clinical settings. Home use is recommended under the guidance of a therapist or healthcare provider.
4. What does 'Class I physical therapy equipment' mean?
Class I indicates a low-risk medical device classification for general physical therapy equipment. Exact regulatory requirements vary by country; check local regulations and product documentation for compliance details.
5. What are the device's weight and size specifications?
The product has a listed gross weight of 5.500 kg and is described as 'universal' size. For precise physical dimensions or fit for a specific user, contact the manufacturer or check the product manual.
6. Is the TJ-HE001 adjustable to different patient needs?
Yes. The device includes adjustable settings to personalize rehabilitation parameters and fit, allowing customization for different patients and therapy requirements.
7. Does the device require a power source or batteries?
Power or battery details are not specified in the provided information. Contact the manufacturer or supplier to confirm whether the TJ-HE001 is passive, electrically assisted, or requires a power source and to get specifications.
8. How is the device cleaned and maintained?
For routine cleaning, use a soft cloth and a mild disinfectant or soap solution; avoid soaking or immersing the device. Do not use harsh chemicals or abrasive cleaners. Refer to the product manual for detailed maintenance, inspection and storage instructions.
9. Are there any contraindications or safety precautions?
Precautions include consulting a physician or therapist before use. The device should not be used on patients with unhealed fractures, active infections, severe pain of unknown cause, or other conditions where motion is medically contraindicated. Follow clinician direction and the user manual for safe operation.
10. Does the TJ-HE001 come with training or technical support?
Online technical support is provided for users. For training, ask your supplier or clinician if they offer setup assistance, instructional materials or supervised introduction sessions.
11. What accessories or components are included with the device?
The included accessories are not listed in the provided description. Check the product listing or contact the seller/manufacturer for a full packing list and available optional accessories.
12. How long before patients can expect to see improvement?
Rehabilitation timelines vary widely depending on the individual, severity of impairment, frequency of therapy and overall health. The device is a tool to assist therapy; expected outcomes and timelines should be discussed with the treating rehabilitation professional.
13. Is the device appropriate for children or small adults?
The product is listed as 'universal' size but specific pediatric suitability is not specified. Contact the manufacturer or your clinician to confirm fit and safety for children or small adults.
14. What warranty and return options are available?
Warranty and return policy information are not included in the basic product description. For warranty length, coverage and return terms, contact the seller or manufacturer directly.
15. How do I get more detailed technical or regulatory information?
For detailed specifications, user manuals, regulatory certificates or clinical use guidance, contact the manufacturer or authorized distributor. They can provide technical sheets, certification documents and clinical support materials.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading