B21, China Town Mall, Midrand
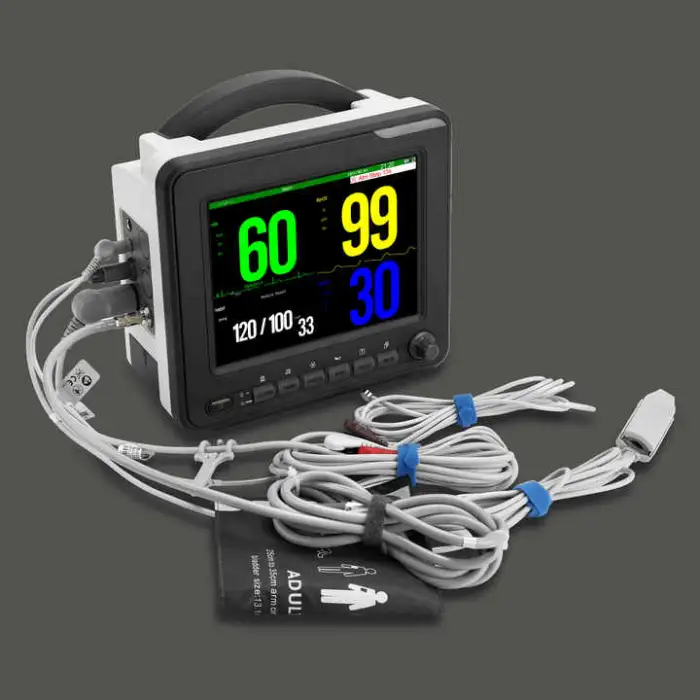
SNP9000L Multi-Parameter Patient Monitor Clinical Analytical Instrument
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1601460084156

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 23 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
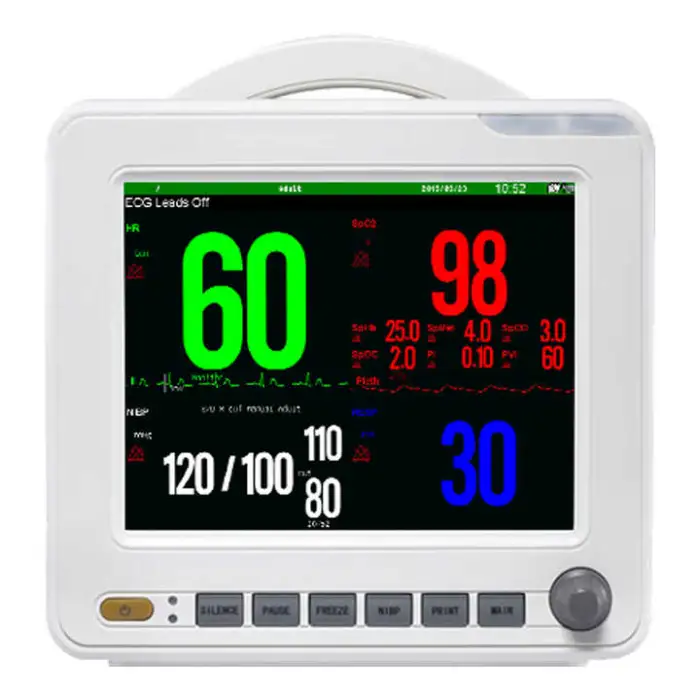
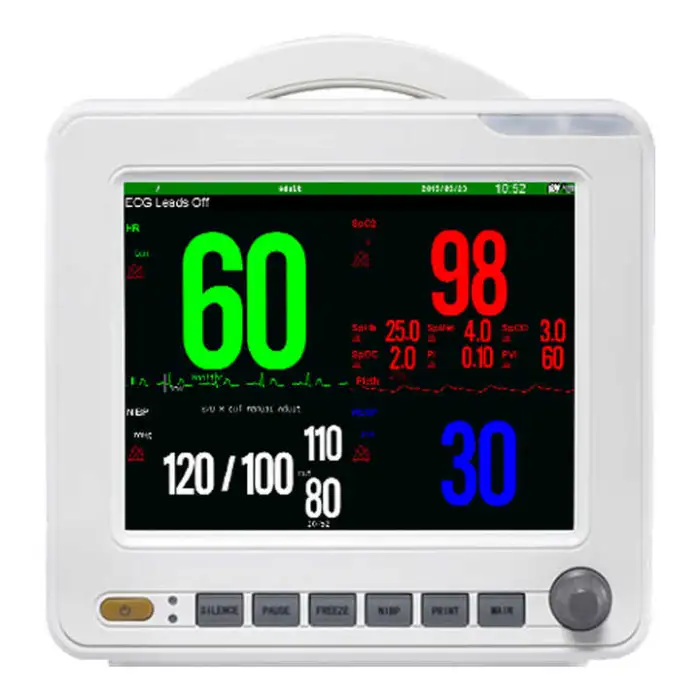
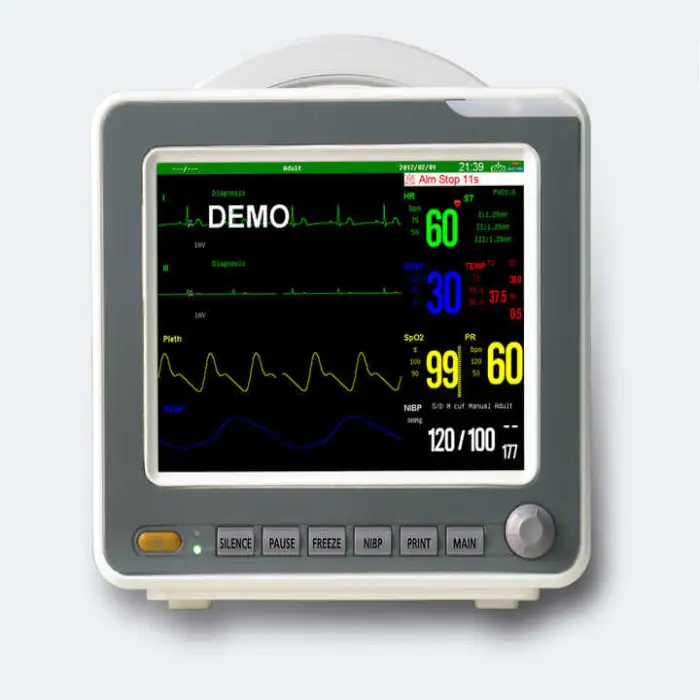
1. What vital signs and parameters does the SNP9000L monitor?
The product description specifically lists ECG, NIBP (non-invasive blood pressure) and SpO2. Other parameters are not specified; consult the vendor for an exact list of supported parameters or optional modules.
2. What kind of display does the SNP9000L have?
It features a high-resolution 18-color TFT display with 800 x 600 dpi and selectable colors for parameter waveforms and characters for clear, customizable viewing.
3. What SpO2 features are included?
The SNP9000L includes advanced SpO2 features such as pitch tone variation and drug dose calculation, per the product description.
4. What does 'Separated Para Board' mean on this monitor?
The monitor uses separate parameter boards/modules for ECG, NIBP and SpO2. This typically makes servicing and module replacement easier; contact the supplier for details on modularity and spare boards.
5. Which clinical settings is the SNP9000L suited for?
It is described as ideal for hospitals, clinics, emergency care settings, outpatient facilities and critical care situations where continuous monitoring of vital signs is required.
6. What regulatory or classification information is provided?
The product is listed as Instrument Classification: Class II. For country-specific regulatory compliance (FDA, CE, etc.), check with the manufacturer or distributor.
7. What warranty and technical support are provided?
The specification lists 2 years of online technical support. For details on coverage, response times, and options for onsite service or extended warranties, contact the vendor.
8. What are the package size and weight?
The listed package size is 15 x 15 x 15 cm and gross weight is 5.000 kg. If you need the unit's physical dimensions (chassis size) or net weight, confirm with the manufacturer, as the provided values refer to packaging.
9. Does the SNP9000L have a battery backup or internal battery?
The product description does not specify battery or power backup. If battery operation or UPS capability is required, please ask the supplier for the exact power specifications and available battery options.
10. What accessories and consumables are included with the monitor?
The listing only states 'selling units: single item' and does not enumerate included accessories. Typical accessories for multi-parameter monitors may include ECG lead sets, NIBP cuff, and SpO2 sensor, but you should confirm the exact items supplied with your purchase.
11. Does the SNP9000L support networking or interoperability with hospital systems (e.g., HL7, EMR)?
Network and interface capabilities are not specified in the provided description. Contact the manufacturer or distributor to confirm available communication ports, protocols and integration options.
12. What user interface languages and training are available?
The product description does not list supported languages or training offerings. Ask your vendor about available user-interface languages, on-site or online training, and user manuals.
13. How should the SNP9000L be cleaned and disinfected?
Specific cleaning instructions are not provided. Follow the manufacturer's cleaning and infection-control guidelines. As a general rule, use approved medical-grade disinfectants, avoid excessive liquid ingress into ports, and disconnect sensors/cables per the manual before cleaning.
14. What maintenance and calibration are required?
Routine maintenance and periodic calibration are typically required for clinical monitors, but the description doesn't specify intervals. Use the 2-year online technical support to obtain maintenance schedules, recommended calibration intervals and authorized service providers.
15. How do I purchase, get a quote, or request technical documentation?
The product listing does not include sales contact details. Contact the manufacturer (Brand: CAH) or your authorized distributor to request pricing, availability, technical specifications, user manuals, and CE/FDA documentation if needed.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading