B21, China Town Mall, Midrand
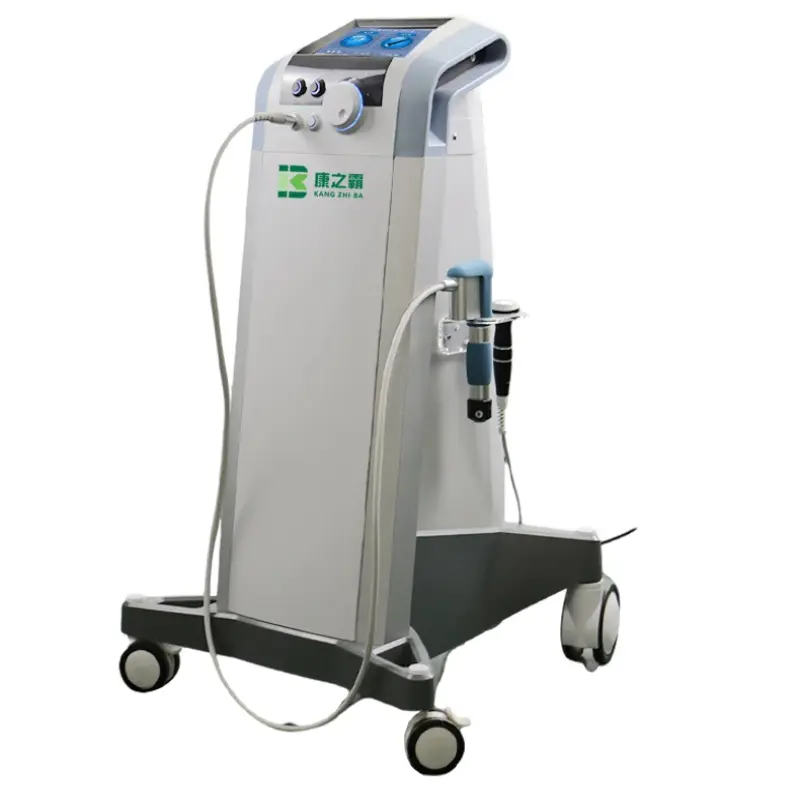
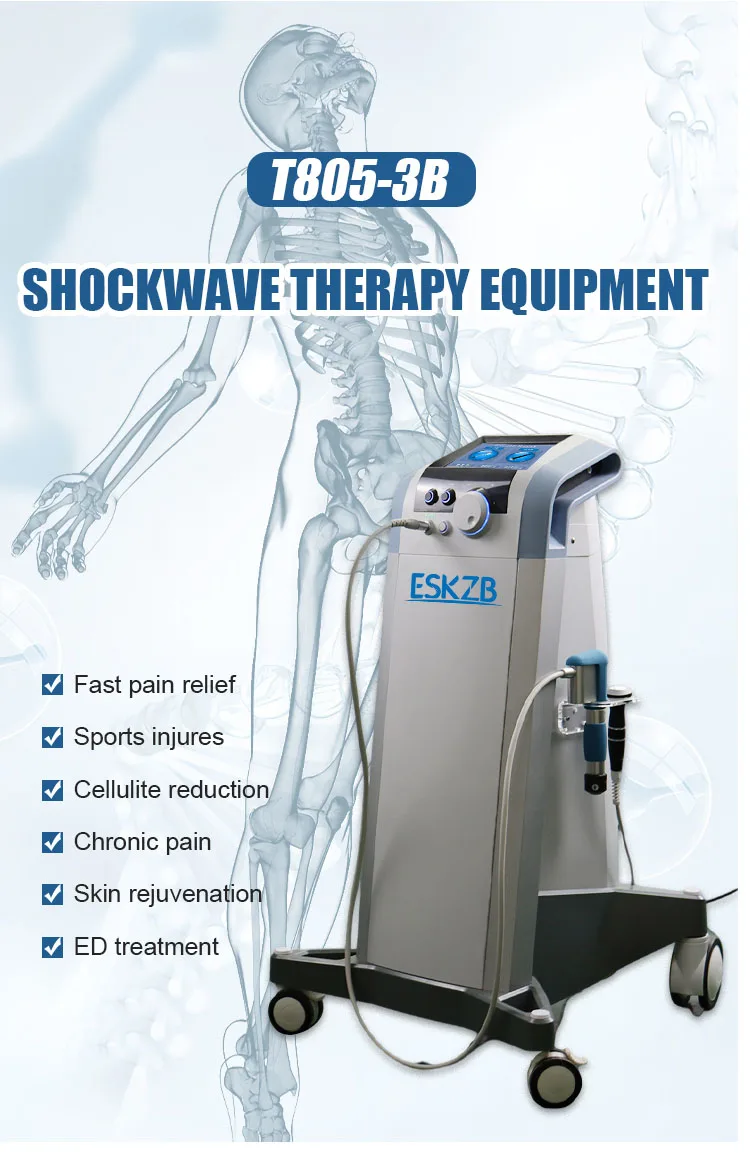
Shockwave machine 10 bar tissue repair therapy machine electro-magnetic shockwave therapy
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1601033821930

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 22 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Shockwave Machine 10 Bar Tissue Repair Therapy Machine?
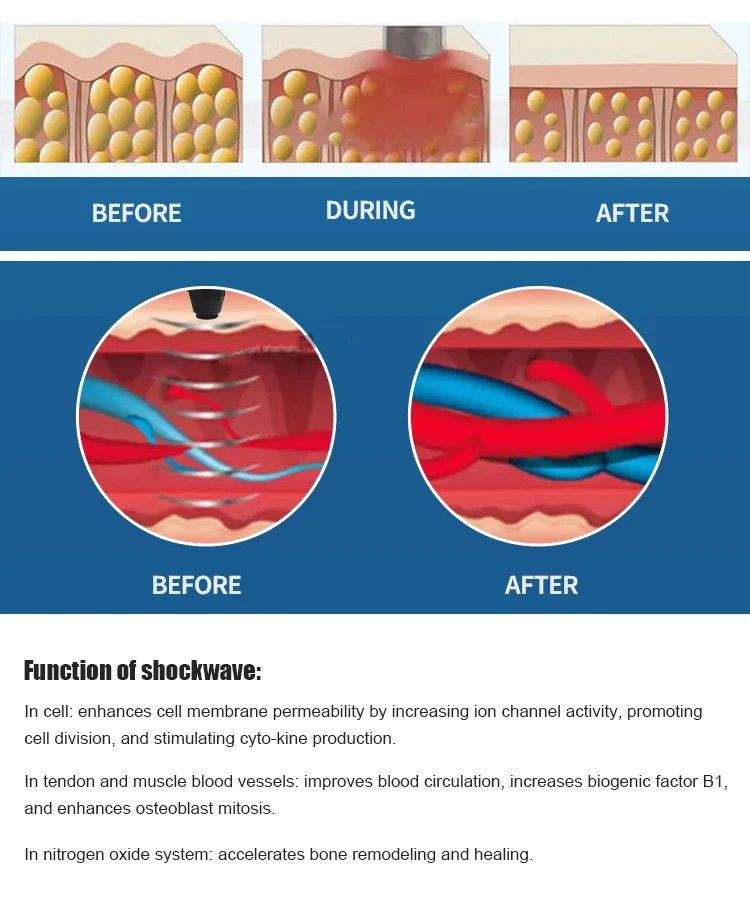
It is an advanced electromagnetic shockwave therapy device designed for physical therapy professionals to treat musculoskeletal conditions and support tissue repair using focused mechanical pulses.
2. How does this shockwave therapy equipment work?
The device generates high-energy mechanical (acoustic) pulses delivered through therapy heads to targeted tissues. These pulses stimulate healing responses, improve blood flow, and reduce pain in musculoskeletal areas.
3. What does '10 bar' mean?
The '10 bar' specification refers to the device's adjustable energy setting range, which in this model goes from 0.5 to 10 bar, allowing clinicians to select appropriate intensity for different clinical needs.
4. What frequency range does the equipment offer and why does it matter?
The unit operates between 1–21 Hz. Frequency affects the number of shock pulses delivered per second; lower frequencies are often used for focused, higher-energy treatments while higher frequencies allow faster delivery at lower energy per pulse. Choice depends on treatment protocol.
5. What clinical conditions can be treated with this machine?
Common indications include tendinopathies (e.g., plantar fasciitis, Achilles tendinopathy), myofascial trigger points, calcific shoulder tendinitis, and other soft-tissue and chronic pain conditions. Treatment should follow clinical guidelines and individual assessment.
6. How many therapy heads are included and what are they for?
The system includes seven physical therapy heads designed to treat different areas and tissue depths. Heads vary in shape and contact area to allow treatment of focal points, broad areas, and different tissue types.
7. What power and voltage specifications does the device have?
The device has a maximum power output of 350W and is compatible with both 220V/50Hz and 110V/60Hz mains supplies, making it suitable for use in many regions.
8. How long is a typical treatment session and how many sessions are recommended?
Session length and number depend on the condition and protocol; typical sessions range from 5 to 20 minutes with multiple sessions often scheduled over several weeks. Follow the device manual and clinical best practices for specific protocols.
9. Is the machine portable?
The unit is reasonably portable for clinic use with a gross weight of 52 kg and compact outer packaging (61 × 61 × 112 cm). It is robust but may require assistance to move safely.
10. What safety considerations or contraindications should I be aware of?
Contraindications commonly include pregnancy, active infection or malignancy at the treatment site, coagulation disorders or use of anticoagulants, and implanted electronic devices such as pacemakers near the treatment area. Always review the user manual and consult a physician when in doubt.
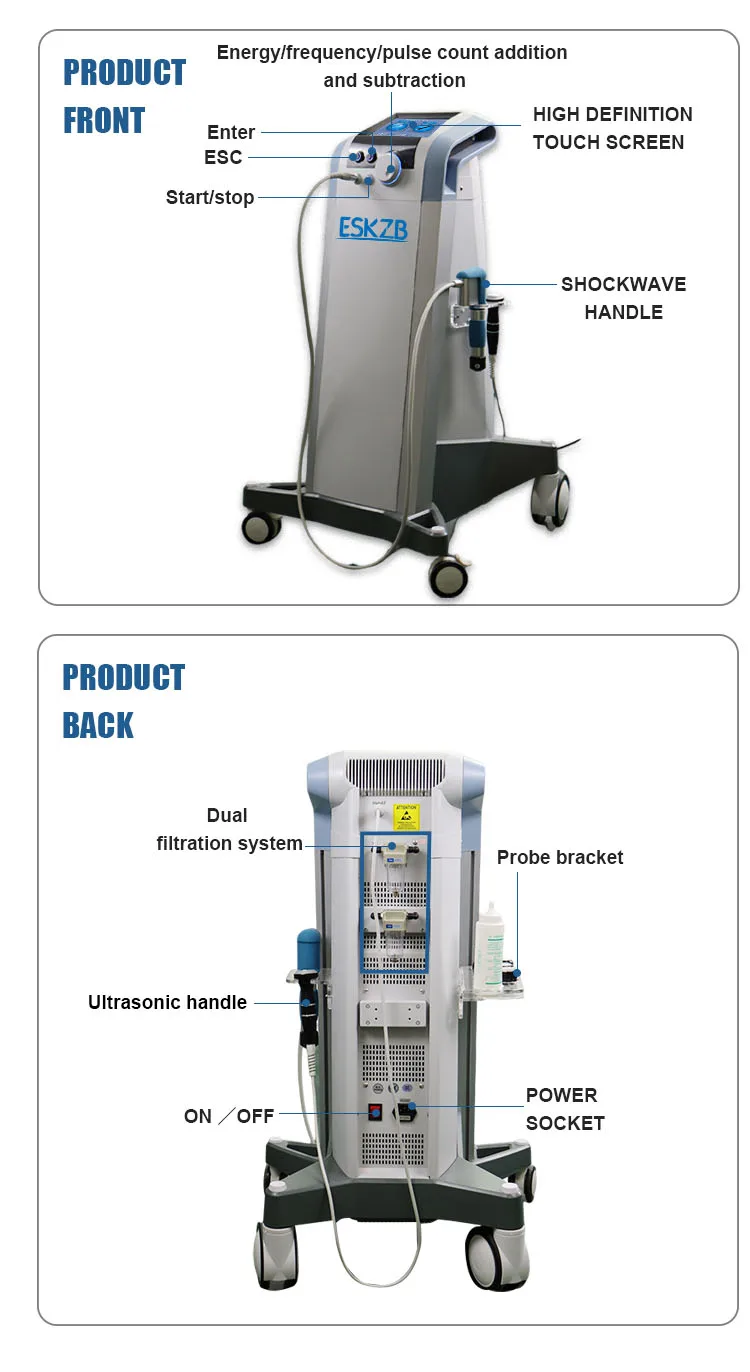
11. How is the device operated and how user-friendly is it?
The device features a 10-inch touch screen with an intuitive interface for easy navigation and control of energy, frequency, and treatment parameters.
12. What maintenance and cleaning are required?
Regular maintenance includes inspecting therapy heads and connections, keeping the unit clean and dry, and following manufacturer instructions for cleaning contact surfaces with approved disinfectants. Routine checks and servicing per the manual extend device life and safety.
13. What accessories or consumables come with the machine?
The system includes seven therapy heads and standard packaging. Additional consumables or optional accessories (e.g., protective covers, spare heads) may be available—check with the supplier for a complete list.
14. Is training required to use the machine and is training available?
Operators should be trained in shockwave therapy principles and the device's specific settings and safety procedures. Many suppliers offer initial training, user manuals, and clinical guidelines—confirm available training with the vendor.
15. What warranty and support options are available?
Warranty and after-sales support vary by seller and region. Contact the supplier or manufacturer for details on warranty length, coverage, technical support, and spare parts availability.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading