B21, China Town Mall, Midrand
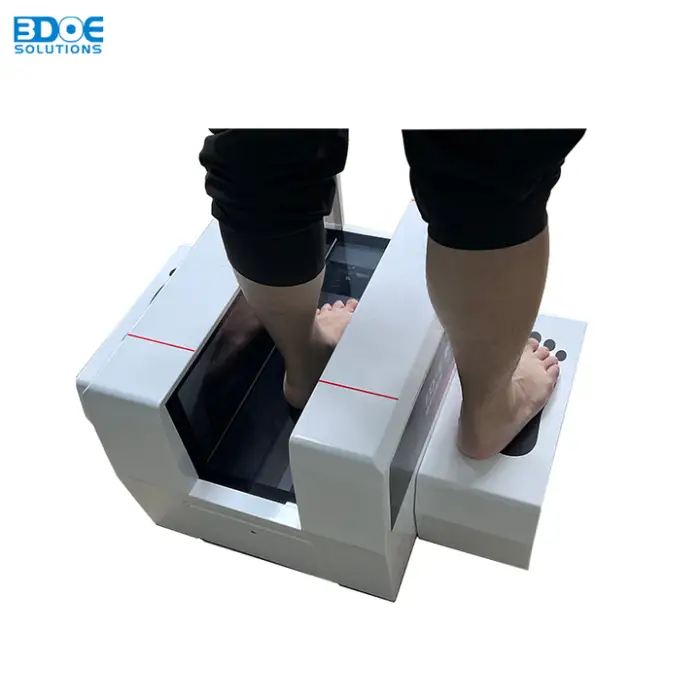
Orthotic Custom Foot Scanner: Detailed Foot Shape and Pressure Measurement
- Section : Medical Supplies
- Category : Medical Consumables
- SKU : 1601157615703

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 23 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What does the Orthotic Custom Foot Scanner measure?
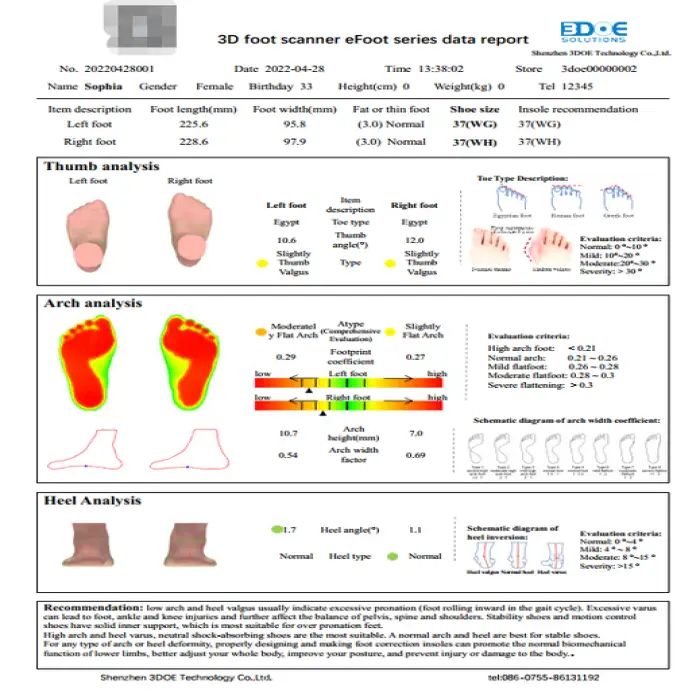
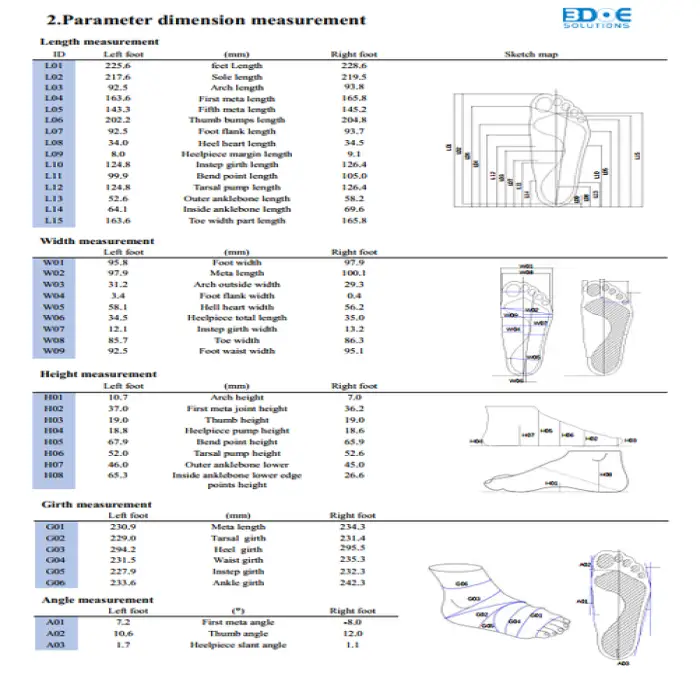
It captures detailed 3D foot shape and plantar pressure distribution, producing a high-resolution model used for custom orthotic design and fit assessment.
2. How long does a scan take?
Each single-foot scan takes under 10 seconds, allowing fast throughput in clinic or retail settings.
3. How accurate are the scans?
The scanner has a stated scanning error of ±0.5 mm, providing clinically useful precision for orthotic manufacturing and assessment.
4. What scanning technology is used and is it safe?
It uses non-contact line laser scanning (single foot scan). The non-contact approach is safe and hygienic for patients and customers.
5. What is the maximum foot size the device can scan?
The scanning range is 350 × 170 × 150 mm (length × width × height). Feet exceeding these dimensions may not be fully captured; check with the vendor for accommodation options.
6. Can I scan both feet at once?
The device performs single-foot scans. To capture both feet, perform two separate scans—one per foot—and combine the data in the analysis software if needed.
7. What are the power requirements and can it be used internationally?
The unit requires AC 220V power. For regions with different mains voltages, use an appropriate external voltage converter or request a region-specific model from the supplier.
8. Which file formats and software integrations are supported?
Common practice is to export 3D models and pressure data in standard formats (for example STL/OBJ/PLY for meshes and CSV/image formats for pressure maps). Exact supported formats and available SDK/API or EMR integrations should be confirmed with the manufacturer or reseller.
9. Can the scanner produce pressure maps for gait or static analysis?
Yes — the product description includes pressure measurement capability for assessing plantar pressure. For dynamic gait analysis capability and sampling rate details, verify the specific software/firmware options with the vendor.
10. Is the device portable and how much does it weigh?
The scanner has a compact design for easier storage and transport, but it has a gross weight of 35.000 kg and packaged dimensions of 73 × 46 × 44 cm. It is movable but not handheld; consider two-person handling or a cart for transport.
11. Are OEM and ODM customization options available?
Yes — the product offers OEM and ODM options. Contact the manufacturer to discuss branding, hardware or software customization, and minimum order requirements.
12. Where is this scanner typically used?
Typical applications include clinics for custom orthotics, shoe stores offering personalized footwear, sports performance centers, rehabilitation facilities, and research labs studying foot health.
13. What maintenance, calibration and cleaning are required?
Routine maintenance includes keeping optics and the scanning area clean, following manufacturer cleaning instructions (non-abrasive, lens-safe cleaners), and periodic calibration per the supplier’s schedule. Ask the vendor for an official maintenance and calibration plan.
14. How much training is needed to operate the scanner?
The scanner is designed to be user-friendly, but staff training is recommended to ensure correct patient positioning, optimal scan quality, software operation, and interpretation of results. Training options are typically available from the manufacturer or reseller.
15. What warranty, certifications or regulatory approvals does the product have?
Warranty terms, certifications (CE, FDA, etc.), and regulatory approvals depend on the model and region. Contact the manufacturer or your reseller for detailed warranty coverage and copies of relevant certifications before purchase.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading