B21, China Town Mall, Midrand
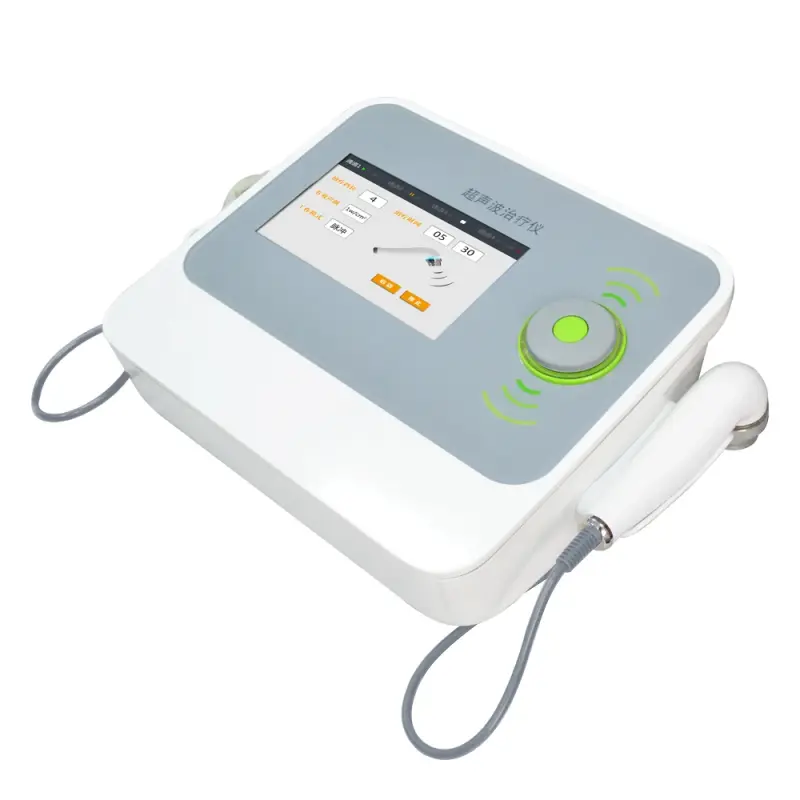
MY-S130H-2: Portable Medical Ultrasound Therapy Device
- Section : Medical Supplies
- Category : Physical Therapy Equipment
- SKU : 1600432824753

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 19 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Mayamed MY-S130H-2?
The Mayamed MY-S130H-2 is a Class II portable medical ultrasound therapy device designed to reduce pain and promote healing in soft tissues. It is intended for use in rehab centers, hospitals and, with appropriate guidance, home health care.
2. How does this device relieve pain and aid recovery?
The device delivers therapeutic ultrasound waves that can increase local blood flow, reduce muscle tension, and support tissue healing. It provides a non‑invasive approach to address muscular pain, joint discomfort and soft tissue injuries. Clinical use and results depend on the condition treated and treatment parameters.
3. Is the MY-S130H-2 a shockwave device or an ultrasound device?
The product is described as a therapeutic ultrasound device; some materials also reference shockwave therapy concepts. If you need a dedicated shockwave (radial/focused) system, confirm the exact therapy modality and technical specifications with the manufacturer or the device manual before purchase.
4. Where can I use the MY-S130H-2?
It is designed for use in rehabilitation centers, hospitals and home settings. Home use should be done under the direction of a qualified healthcare professional and after learning proper application techniques from the user manual or clinician.
5. Who is qualified to operate this device?
Healthcare professionals (physiotherapists, physicians, trained clinicians) should operate the device for clinical treatments. Patients may use it at home if trained and following a healthcare provider's instructions and the device's user manual.
6. What are the main specifications and physical features?
Model: MY-S130H-2. Product type: medical ultrasound therapy device. Material: durable ABS housing. Color: white. Function: reduces pain and promotes healing. For full technical specs (frequency, intensity, power requirements) consult the product manual or manufacturer.
7. Are there any contraindications or precautions?
Yes. Common contraindications for therapeutic ultrasound include use over malignancies, active infections, open fractures or wounds, areas with impaired sensation, over the eyes or reproductive organs, and directly over implanted electronic devices (e.g., pacemakers). Do not use over areas with deep venous thrombosis or hemorrhagic conditions. Always consult the device manual and a healthcare professional before use.
8. Can pregnant people use the device?
Therapeutic ultrasound is generally contraindicated over the abdomen and pelvic regions during pregnancy. Pregnant people should consult their healthcare provider before any treatment.
9. Is it safe to use on children?
Use on children should be performed only under the supervision of a qualified healthcare professional. Dosage and treatment parameters will differ from adults and should be prescribed by a clinician.
10. How long is a typical treatment session and how often should I use it?
Treatment duration and frequency vary with the condition and clinician guidance. Typical sessions for therapeutic ultrasound often range from about 5 to 15 minutes per treatment area, with treatments commonly repeated 1–3 times per week. Follow the treatment plan prescribed by your healthcare provider and the instructions in the user manual.
11. What power source does the device use and is it rechargeable?
The product description does not specify power source or battery details. Because it is portable, it may be mains-powered or battery/rechargeable. Check the product manual or contact the seller/manufacturer to confirm power requirements and battery options.
12. How should I clean and maintain the MY-S130H-2?
Wipe the ABS housing and probe with a soft cloth dampened with mild detergent or a manufacturer‑approved disinfectant. Do not immerse the device or probe in liquid. Inspect cables and connectors regularly for damage. Refer to the user manual for specific cleaning agents, probe care and storage recommendations.
13. What accessories come with the device?
The description does not list specific accessories. Typical packages for ultrasound therapy devices may include the treatment probe/transducer, power adapter or cable, coupling gel, user manual and a carrying case. Confirm included items with the seller or product listing.
14. What warranty, service or technical support is available?
Warranty and service terms are not provided in the basic description. Check with the seller or manufacturer for warranty length, what it covers, and how to obtain repair or technical support. Keep purchase documentation for warranty claims.
15. Does the device have regulatory certifications?
The product is described as a Class II medical device, indicating regulatory classification in some jurisdictions. For specific certifications (e.g., FDA 510(k), CE marking, local regulatory approvals), consult the manufacturer or product documentation and verify compliance with local regulations before clinical use.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading