B21, China Town Mall, Midrand
Extracorporeal Shockwave Therapy Machine HUMAIN Ultrasound Therapy Lithotripter
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1600953489858

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 23 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
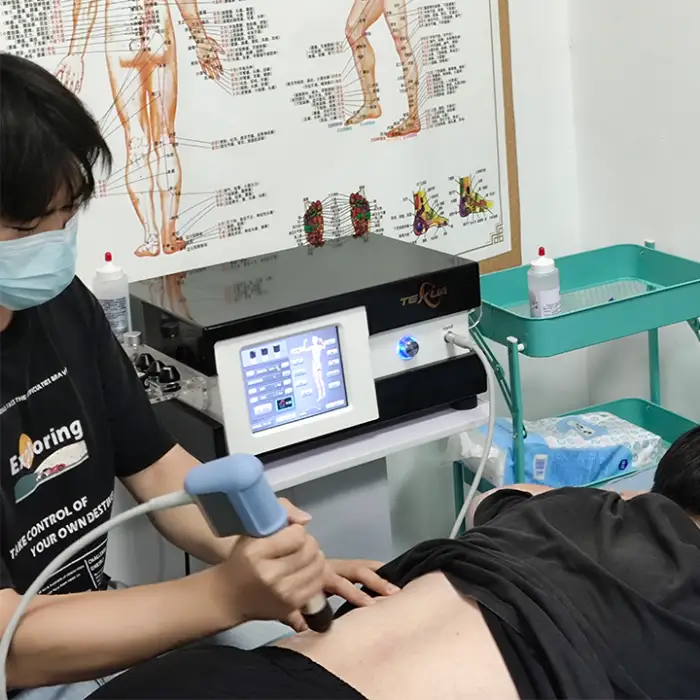
1. What is the Extracorporeal Shockwave Therapy Machine HUMAIN Ultrasound Therapy Lithotripter?
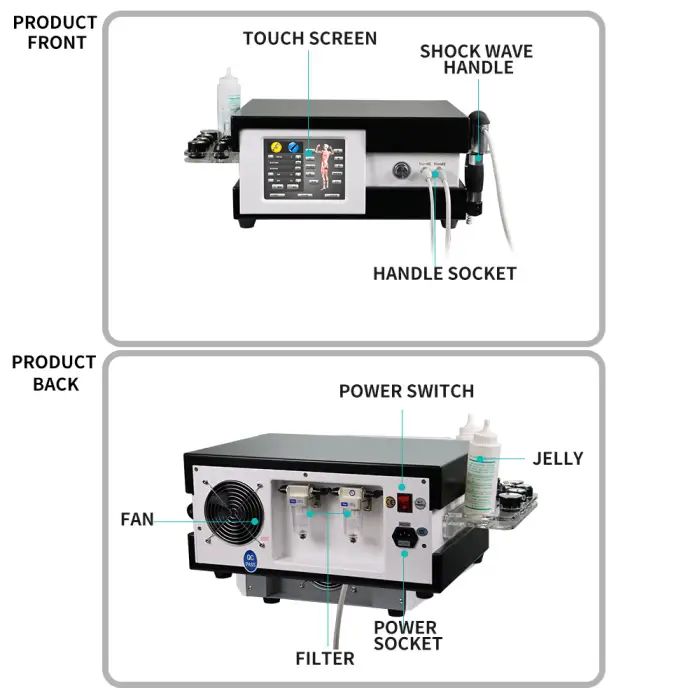
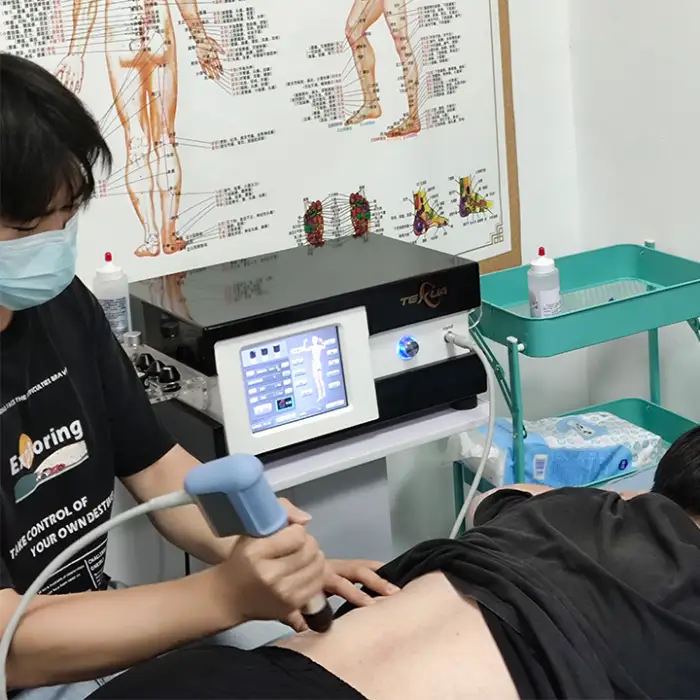
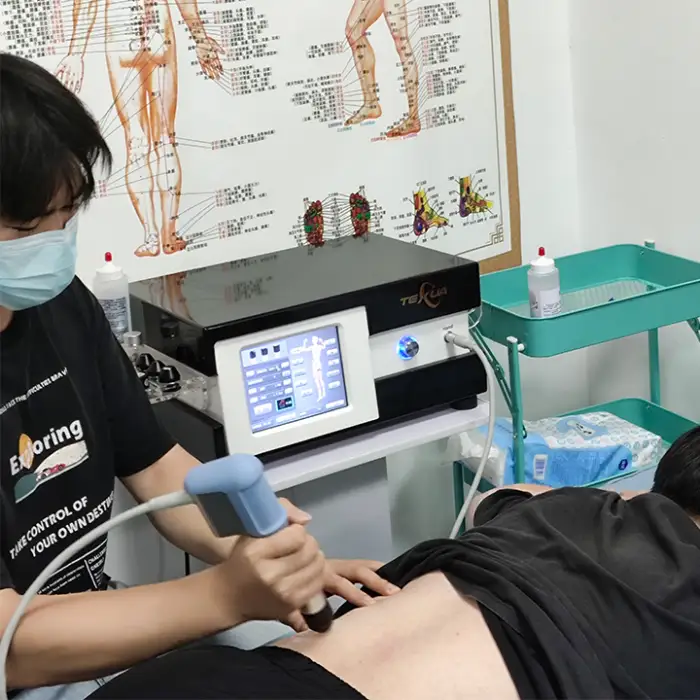
It is a medical physiotherapy device (Brand: Texua, Model: T502+2) designed to reduce pain and support functional rehabilitation using extracorporeal shockwave/ultrasound therapy. It is intended for use in clinics and health recovery centers.
2. What are the main specifications of this device?
Instrument classification: Class IIF. Frequency range: 1–21 Hz. Power: 380 W. Weight: 17 kg. Voltage: AC 110–220V, 50–60Hz. Brand: Texua. Model: T502+2.
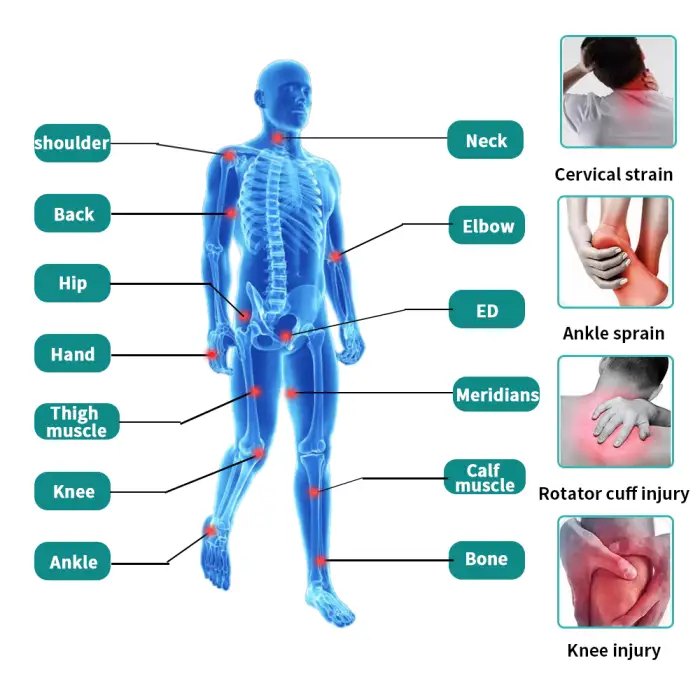
3. What clinical applications is this machine suitable for?
It is used for musculoskeletal pain reduction and functional rehabilitation in physiotherapy settings, including hand rehabilitation and a variety of clinic-based recovery treatments. Specific treatment decisions should be made by qualified clinicians.
4. How does the adjustable frequency benefit treatment?
Adjustable frequency (1–21 Hz) allows clinicians to tailor the therapy intensity and treatment parameters to the patient’s condition and tolerance, which helps optimize outcomes for different indications.
5. Is the device portable?
Yes. At 17 kg the unit is lightweight compared with many clinical systems, making it easy to move within clinics and recovery centers. Confirm local transport and mounting options with the supplier.
6. What power supply is required?
The device operates from AC 110–220V at 50–60Hz, allowing use in regions with either voltage standard without an external transformer.
7. What accessories and spare parts are provided?
The product description states it comes with free spare parts and OEM service. For a complete list of included accessories (applicators, cables, stands, etc.) and spare parts, contact the supplier or refer to the packing list.
8. Is operator training required?
Yes. The device should be operated by trained healthcare professionals. Training ensures correct parameter selection, safe application, and optimal patient outcomes. Ask the supplier about training options.
9. Are there contraindications or precautions?
There are common contraindications for shockwave/ultrasound therapy (for example, pregnancy, certain bleeding disorders, or active malignant lesions). Always consult the device manual and a qualified clinician to review patient-specific contraindications before treatment.
10. What safety and user controls does the machine offer?
The device provides adjustable treatment parameters (frequency and intensity) so clinicians can control therapy dosing. Follow the user manual for recommended protocols, safety checks, and emergency procedures.
11. How should the device be maintained and cleaned?
Regular maintenance should follow the manufacturer’s instructions. Keep the unit clean, inspect cables and applicators before use, and perform periodic functional checks. For servicing or parts replacement, use authorized service channels.
12. What warranty and after-sales support are available?
The listing mentions free OEM service and spare parts. Specific warranty terms, service plans, and response times vary by supplier—contact the seller or Texua representative for full warranty and support details.
13. Can this machine be customized or rebranded for my clinic (OEM)?
According to the product information, free OEM service is available. Contact the supplier to discuss customization, branding, and minimum order requirements.
14. How user-friendly is the device for clinic staff?
The product is described as user-friendly with adjustable settings to simplify treatment setup. Nevertheless, proper training is recommended to ensure safe and effective use.
15. How do I get more detailed technical documentation or ordering information?
For detailed manuals, complete accessory lists, pricing, shipping, and ordering, contact the supplier or authorized Texua distributor. They can also provide technical datasheets and local regulatory information.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading