B21, China Town Mall, Midrand
Effective Portable Varicose Veins Treatment Facial Care Vascular Spider Removal Machine
- Section : Beauty & Personal Care
- Category : Aesthetic medicine
- SKU : 1600074409173

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 21 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
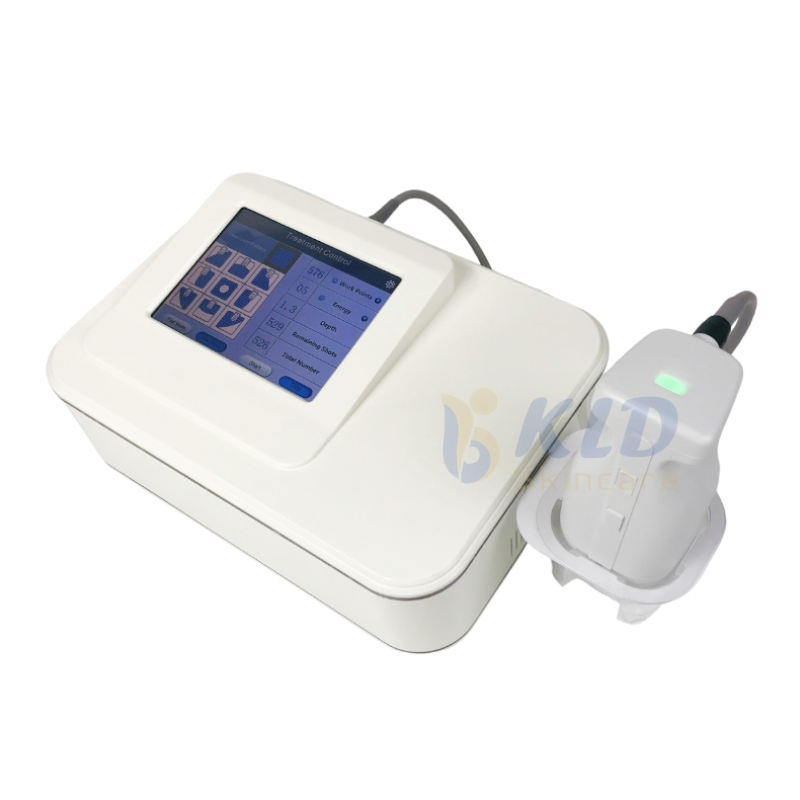
1. What is the Sano RF Vein Removal Device and what does it do?
The Sano RF Vein Removal Device is a portable radio frequency (RF) machine designed for commercial use to treat superficial veins (varicose/spider veins) and provide skin rejuvenation. It uses high-frequency RF energy to coagulate and reduce targeted veins non‑invasively, with adjustable power and pulse/continuous modes for practitioner control.
2. Is this device suitable for home use?
No. The device is intended for professional and commercial use (clinics, medical spas, beauty salons). Proper training and clinical judgment are required to use it safely and effectively.
3. What are the key technical specifications I should know?
Key specs include: operating frequency 30 MHz, adjustable output power 0–100% (±10% tolerance), pulse and continuous work modes, needle diameter 0.01 mm, 10.4‑inch LCD touch screen, dimensions 320×360×370 mm, and weight 7.3 kg. It is CE certified and meets ISO 13485 and ISO 9001 standards.
4. What safety certifications does the device have?
The product description states CE certification and compliance with ISO 13485 and ISO 9001 quality management standards. For regulatory approval beyond these (for example FDA clearance), check with the manufacturer or local distributor.
5. Are there contraindications or clients who should not receive this treatment?
Common contraindications include pregnancy, presence of pacemakers or implanted electronic devices, active skin infections or open wounds at the treatment site, uncontrolled systemic disease (e.g., severe diabetes), and known clotting disorders. Always screen clients and consult the device manual and a medical professional when in doubt.
6. Does treatment with this device hurt and is anesthesia required?
Discomfort varies by client and treatment area. Because the device uses a very fine needle (0.01 mm) and RF energy, many clients experience only mild discomfort or a brief stinging sensation. Topical anesthetic or local numbing can be used according to practitioner preference and local regulations.
7. How many sessions are typically needed to see results?
Results depend on vein size, depth, and individual response. Small spider veins may respond in one or a few sessions, whereas larger or more extensive veins often require multiple treatments spaced weeks apart. Provide clients with individualized expectations during consultation.
8. What are common side effects and risks?
Common temporary side effects include redness, mild swelling, pinpoint scabbing, darkening of treated spots, and slight tenderness. Rare risks include infection, scarring, or undesirable pigment changes if protocols are not followed. Proper technique, post‑care, and sterilization reduce risk.
9. Are the needles or probes reusable and how should they be sterilized?
Follow the manufacturer's instructions. Many practitioners use single‑use sterile needles or disposable tips to minimize infection risk. If reusable components are provided, they must be cleaned and sterilized according to the device manual and local health regulations.
10. What maintenance and cleaning does the device require?
Routine maintenance generally includes cleaning the handpiece and touch screen with approved disinfectants, inspecting cables and connectors, replacing consumables (needles/tips), and storing the unit in a dry environment. Perform functional checks before use and follow the manufacturer's maintenance schedule.
11. What power supply or voltage does the device require?
The product description does not list voltage or plug type. Check the user manual or contact your supplier for local voltage requirements and whether an adapter is needed for your region.
12. Is special training required to operate the device?
Yes. Operators should complete manufacturer training or accredited courses covering device operation, safety protocols, client selection, and emergency procedures. Local regulations may require licensed medical or aesthetic professionals to perform RF vein treatments.
13. What should clients expect for aftercare and downtime?
Aftercare typically includes keeping the area clean, avoiding direct sun exposure, following any topical care instructions, and possibly wearing compression if recommended. Downtime is usually minimal; clients can often resume normal activities the same day, though temporary redness or scabbing may occur.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading







 .
.