B21, China Town Mall, Midrand
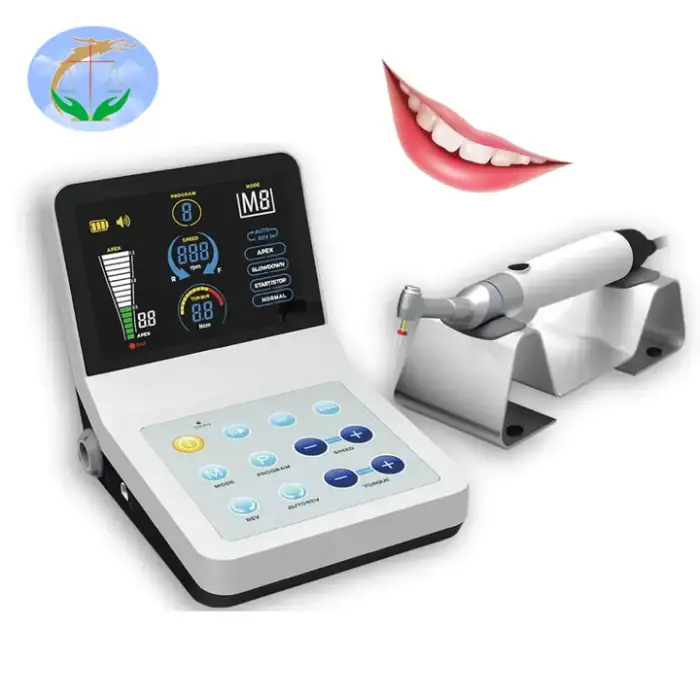
Dental Recipro Endo Motor Dental Endodontic Rotary Machine with Apex Locator Dental Root Canal 2 in 1 Oral Therapy Equipments
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1601266150463

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 23 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Dental Recipro Endo Motor and what does '2 in 1' mean?
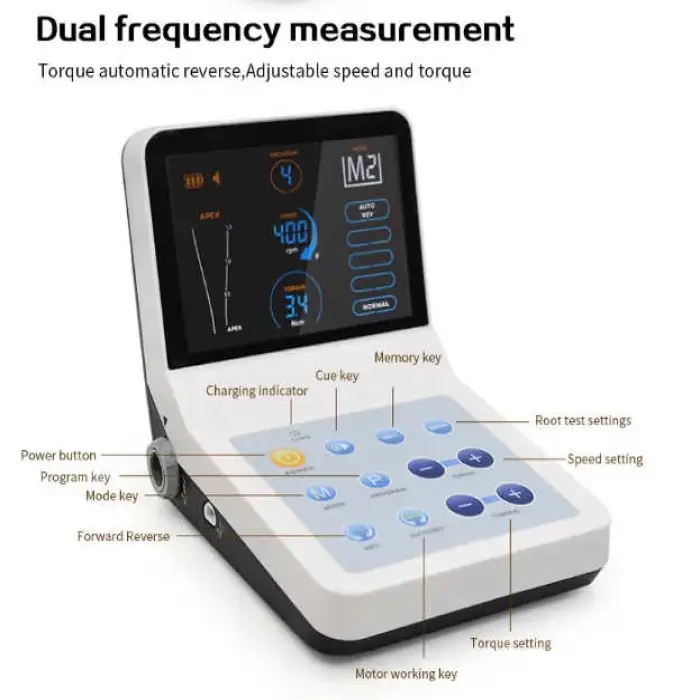
The Dental Recipro Endo Motor (FOREVERMED YJ-1011) is an electric endodontic rotary device that combines a reciprocating/rotary endo motor with an integrated apex locator — allowing both file-driven instrumentation and real-time root apex location in one unit.
2. What are the main features of this endo motor?
Key features include a clear display screen, adjustable torque (0.6–4.0 N·cm), compact lightweight design for easy handling, integrated apex locator, and online technical support.
3. What power source does the device use?
The product is electric-powered. Check the product label or user manual/seller listing for the exact input voltage and plug type for your region.
4. Is the apex locator accurate and how should it be used?
The integrated apex locator provides real-time feedback to assist working length determination. Accuracy can be affected by canal conditions (moisture, metallic restorations, irrigants); always verify with clinical judgment and follow the manufacturer's usage instructions.
5. What torque range does the motor support?
The motor supports an adjustable torque range of 0.6 to 4.0 N·cm to suit different file systems and clinical situations.
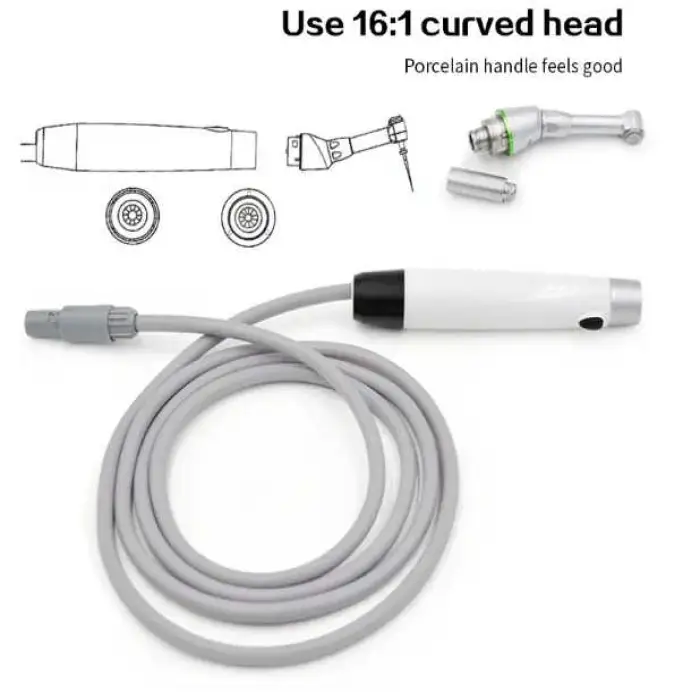
6. Which rotary files are compatible with this motor?
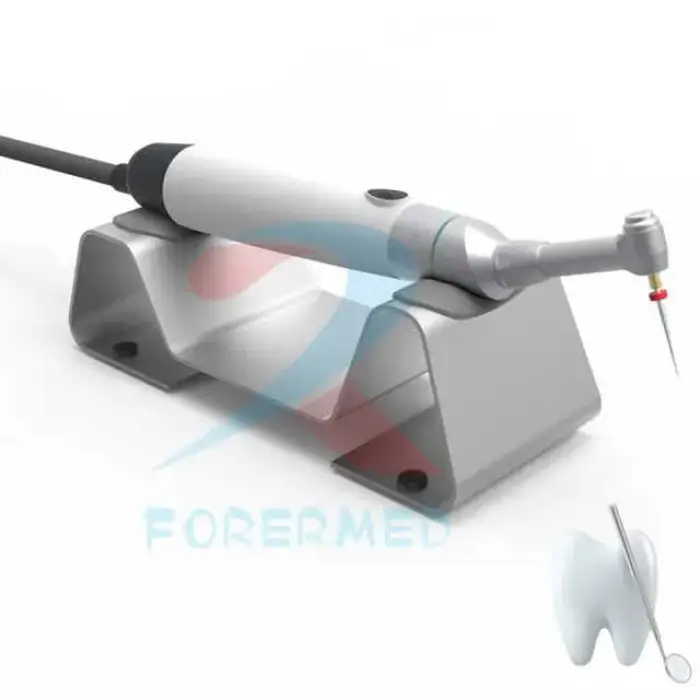
The motor is compatible with standard endodontic rotary and reciprocating file systems. Always confirm compatibility with the file manufacturer and follow recommended speed and torque settings.
7. Is the handpiece and other parts autoclavable?
Sterilization instructions vary by component. Patient-contact items (files, file holders, couplers) should be sterilized or disposed of per infection-control protocols. Consult the user manual or supplier for which specific parts are autoclavable and recommended cleaning procedures.
8. Does the unit come with a foot pedal or other accessories?
Accessory contents are not specified in the general description. Foot pedals, handpieces, contra-angles, and additional accessories may be included in some bundles or sold separately — check the seller's product listing or packing list.
9. What certifications and quality standards does this product have?
The product listing indicates CE certification. For additional certifications or local regulatory compliance, verify with the supplier and local regulatory authority.
10. What is the shelf life and expected service life of the device?
The listed shelf life is 1 year (storage life before sale). Service life will depend on usage, maintenance, and care. For expected operational lifetime, warranty coverage, and maintenance guidance contact the seller or consult the user manual.
11. How do I maintain and clean the device?
Wipe the main unit with a soft, dry or slightly damp cloth; avoid immersion in liquids. Clean and sterilize patient-contact components per manufacturer recommendations. Regularly inspect cables, connections, and moving parts, and seek online technical support if issues arise.
12. Is there technical support or firmware updates available?
The product offers online technical support. For firmware updates or service, contact the manufacturer or authorized distributor as directed in the user manual or support documentation.
13. Are there any safety precautions I should follow?
Follow standard dental device safety: isolate the tooth (rubber dam), avoid use near flammable anesthetics, confirm correct speed/torque settings, do not operate with damaged cables or handpieces, and follow infection-control protocols. Always use clinical judgment alongside device feedback.
14. What are the package dimensions and weight for shipping?
Single package dimensions are listed as 20 x 35 x 15 cm and the gross weight is 8.000 kg.
15. How do I obtain warranty, spare parts, or replacement service?
Warranty terms and spare-part availability vary by seller and region. Check the product listing or contact the seller/manufacturer for warranty details, authorized service centers, and ordering replacement components.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading