B21, China Town Mall, Midrand
Cold Laser Therapy Device (GD10-D) for Effective Treatment
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1600891560712

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 18 Jan, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
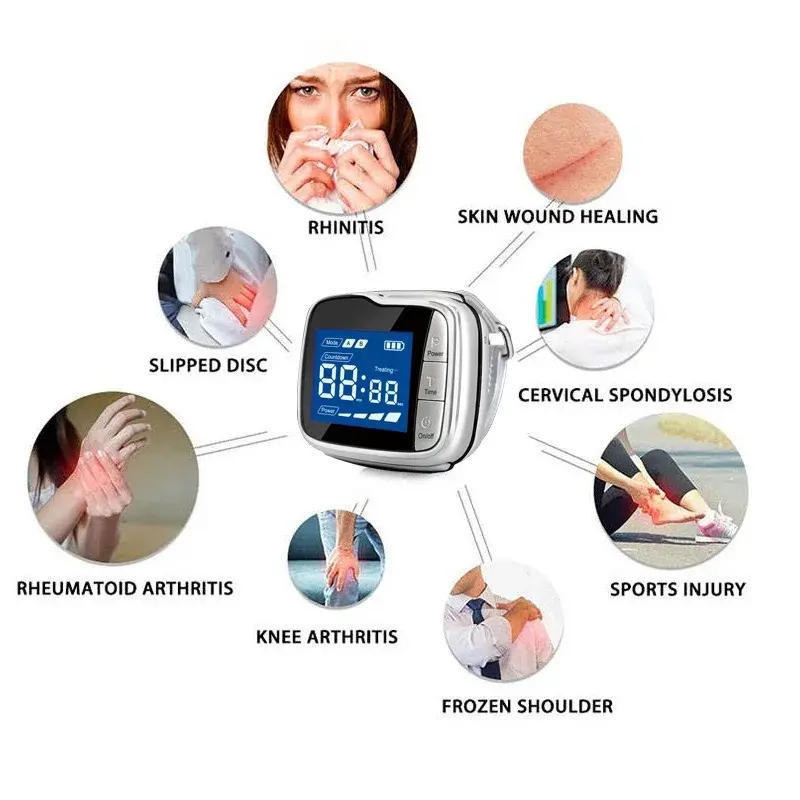
1. What is the Cold Laser Therapy Device (GD10-D) and how does it work?
The GD10-D is a portable low-level laser therapy (LLLT) device that emits 650 nm light via multiple low-power diodes (5 mW each). It delivers photobiomodulation to targeted tissues to support circulation, reduce inflammation, and promote cellular repair. It is intended as a non-invasive adjunctive therapy and should not replace medical treatment prescribed by your healthcare provider.
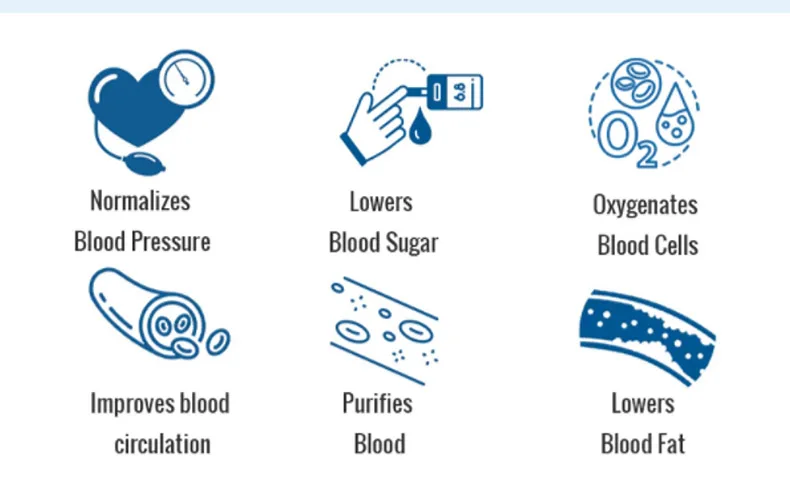
2. Which conditions is the GD10-D intended to help with?
The device is marketed for supportive management of conditions such as diabetic-related blood viscosity issues and high blood pressure, and more generally for improving local circulation and alleviating certain symptoms associated with inflammation or poor microcirculation. Individual responses vary — discuss use with your clinician before starting therapy.
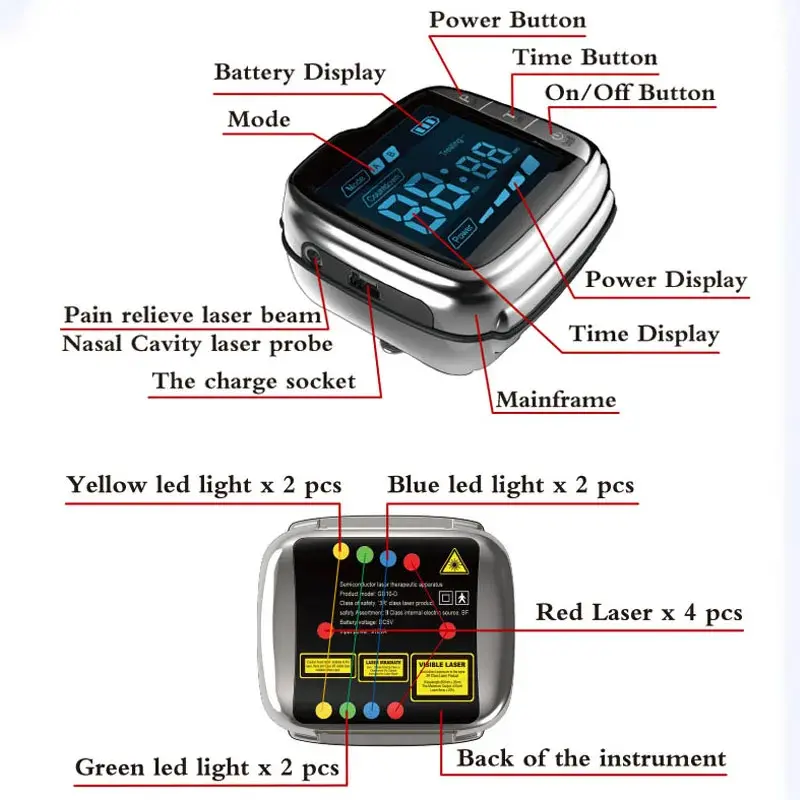
3. What are the main technical specifications of the device?
Key specs: laser wavelength 650 nm, each diode output 5 mW, total of 10 therapy beams plus 1 nasal cavity beam, adjustable treatment time from 15 to 60 minutes, and a built-in 1800 mAh rechargeable lithium battery. The device is CE and CFDA certified.
4. Is the GD10-D safe to use?
The GD10-D uses low-power, non-ionizing lasers and is CE and CFDA certified. When used according to the instructions it is generally considered safe. Important safety measures include avoiding direct eye exposure to the beams, following recommended treatment times, and consulting a healthcare professional if you have serious medical conditions or implanted electronic devices.
5. How do I use the device (basic steps)?
General steps: (1) Read the user manual thoroughly. (2) Clean the treatment area and the device contact surfaces. (3) Power on the device and select the desired treatment time (15–60 minutes). (4) Position the therapy heads over the target area or use the nasal probe as instructed. (5) Wear protective eyewear if recommended and avoid looking into the beams. (6) After the session, power off and store the device. Follow the manual or your clinician's guidance for exact placement and protocol.
6. How long should each treatment session be and how often should I use it?
The GD10-D allows sessions from 15 to 60 minutes. Frequency depends on the condition and clinician guidance — some users start with shorter daily sessions (e.g., 15 minutes) and adjust based on response. Always follow the manufacturer's protocol and your healthcare provider's recommendations.
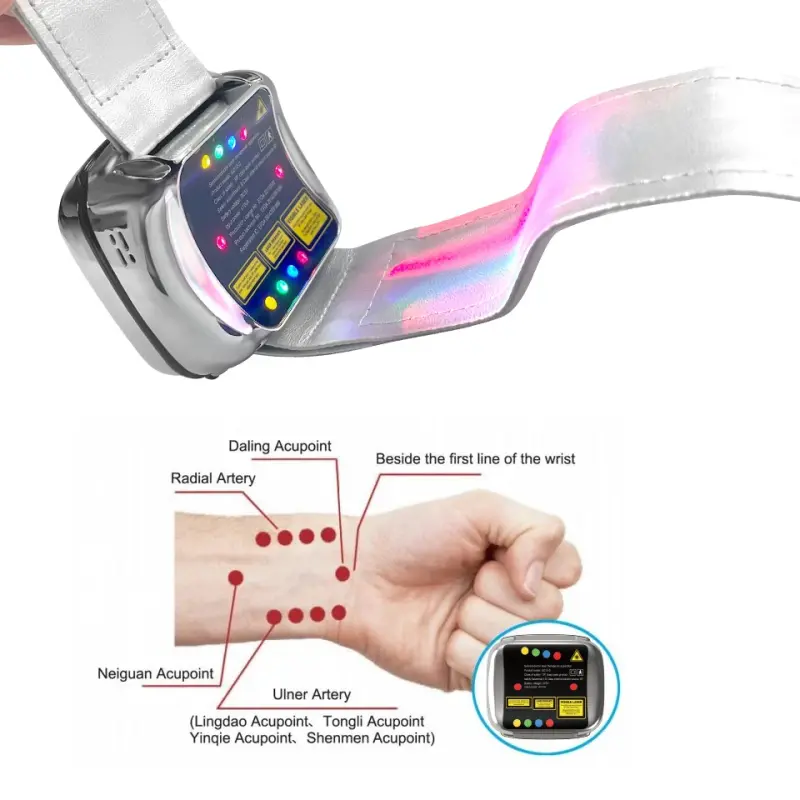
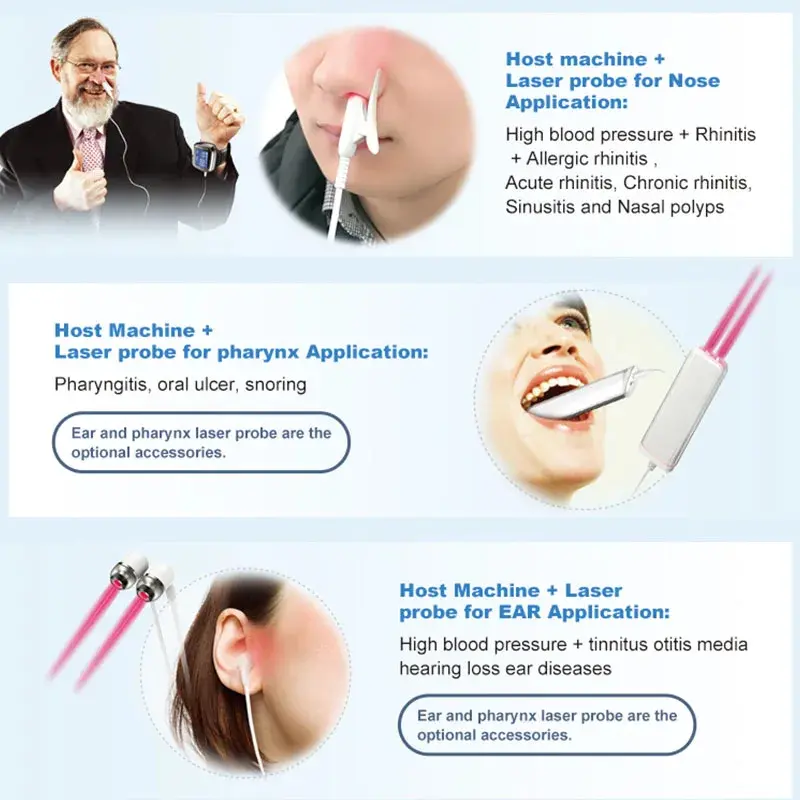
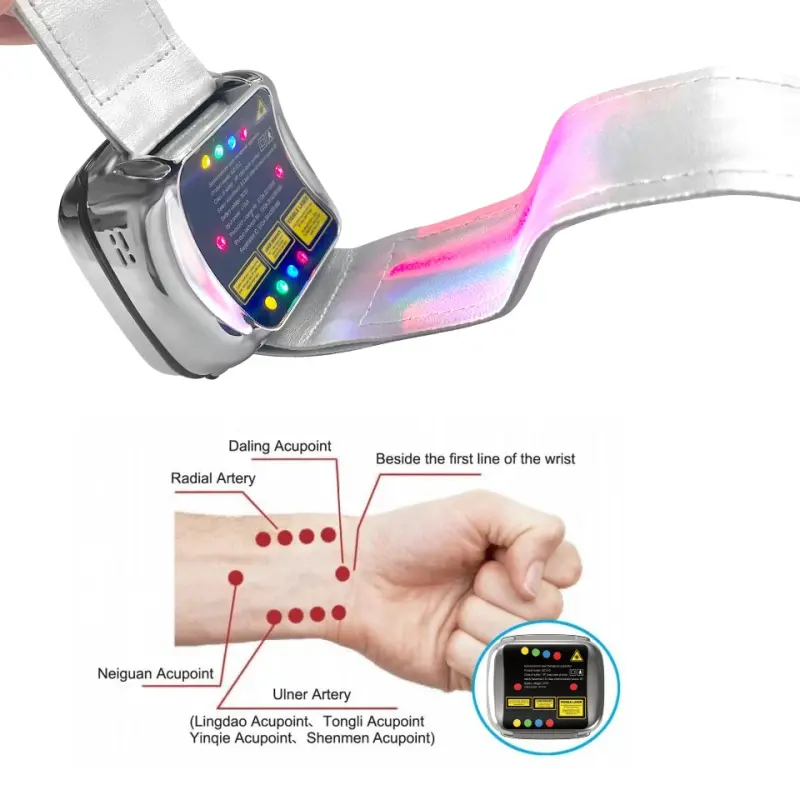
7. Can I use the nasal cavity beam myself?
The device includes a nasal cavity laser beam for intranasal phototherapy. Use it only as directed in the user manual: insert gently, do not force, maintain hygiene of the nasal tip, and follow recommended time limits. If you experience discomfort, stop and consult your healthcare provider.
8. Who should not use this device or should seek medical approval first?
Consult a physician before use if you are pregnant or breastfeeding, have active cancer, epilepsy or photosensitive conditions, major cardiovascular disease, an implanted electronic device (e.g., pacemaker), bleeding disorders, or if you are taking medications that affect photosensitivity or circulation. For children, elderly with cognitive impairment, or any serious medical condition, use only under medical supervision.
9. Are there any side effects I should expect?
Side effects from low-level laser therapy are generally mild and uncommon. Some users report temporary warmth, mild redness, tingling, or transient discomfort at the treatment site. If you experience worsening symptoms, severe pain, skin changes, or other concerning signs, stop use and consult a healthcare professional.
10. Can the GD10-D interfere with my medications or medical devices?
Photobiomodulation itself is non-ionizing and typically does not interact directly with medications, but because it can affect circulation and inflammation, discuss use with your prescriber if you are on blood thinners, antihypertensives, or other critical medications. If you have implanted electronic devices, consult the device manufacturer or your physician before use.
11. How do I clean and maintain the device?
Wipe the external surfaces and probes with a soft cloth slightly dampened with a mild disinfectant or alcohol wipe as instructed in the manual. Do not immerse the device in liquids or use abrasive cleaners. Keep the nasal probe hygienic — use removable tips if provided and replace or disinfect them between users. Store the device in a cool, dry place.
12. How long does the battery last and how do I charge it?
The GD10-D includes a 1800 mAh rechargeable lithium battery designed to support multiple sessions per charge; actual runtime depends on chosen treatment time and settings. Use the supplied charger and follow the manual for charging instructions and safety. If battery performance declines, contact the seller or manufacturer for service options.
13. What certifications does the GD10-D have?
This model is stated to be CE and CFDA certified, indicating compliance with applicable European and Chinese regulatory standards for safety and quality. Certification does not replace medical advice — consult your healthcare provider about whether this device is appropriate for your needs.
14. Is there clinical evidence supporting use of this device?
Low-level laser therapy (photobiomodulation) has a body of scientific literature suggesting benefits for circulation, pain relief, and tissue healing in certain contexts. However, outcomes vary by condition, device parameters, and individual factors. Device-specific clinical trials for the GD10-D may not be available — consult published research and your healthcare provider for evidence relevant to your condition.
15. What warranty, spare parts, and customer support options are available?
Warranty terms, spare parts availability, and customer support vary by manufacturer and retailer. Check the product listing or contact the seller/manufacturer directly for warranty duration, what is covered, how to obtain replacement probes or chargers, and how to get technical or medical usage support. Keep your proof of purchase and the user manual for warranty claims.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals