B21, China Town Mall, Midrand
5KW High-Frequency Portable Digital X-ray Machine - Medical & Veterinary DR
- Section : Medical Supplies
- Category : Veterinary Instrument
- SKU : 1600589408975

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 21 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
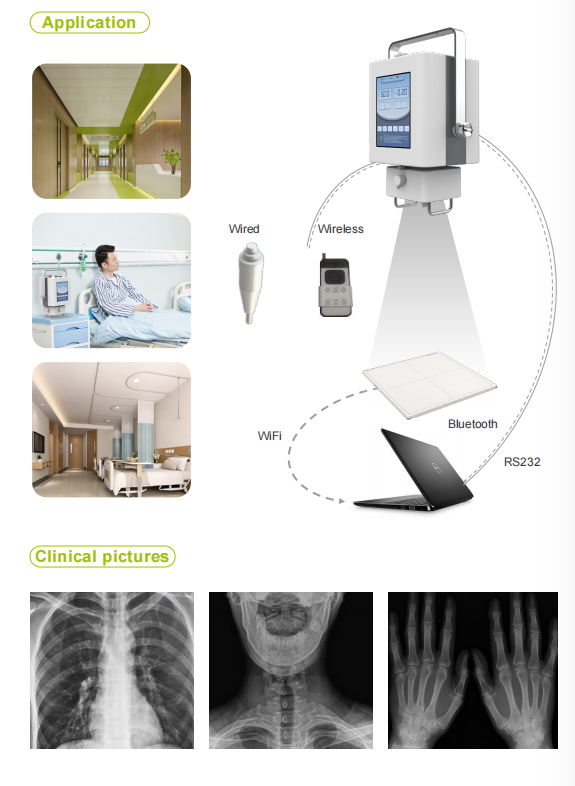
1. What is the 5KW High-Frequency Portable Digital X-ray Machine (MSL MSLGX11)?
The MSL MSLGX11 is a high-frequency radiography machine designed primarily for orthopedic examinations. It is a portable digital X‑ray unit suitable for veterinary clinics, hospitals, and other medical facilities, offering clear radiographic imaging with a compact, durable metal construction.
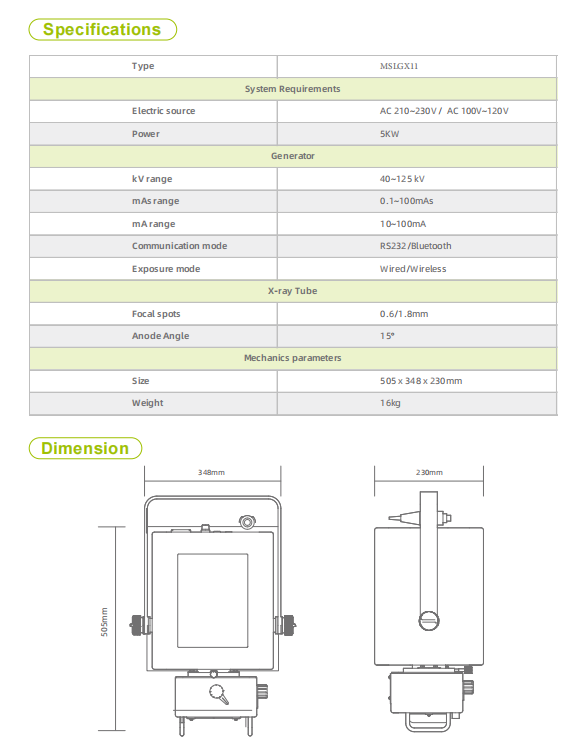
2. What are the key technical specifications?
Key specs include maximum output 90 kV/22 mA, maximum tube current 100 mA, operating frequency 30 kHz, exposure power rating of 2.0 kW, 9‑inch LED numerical display, weight approximately 18.8 kg, and AC power supply AC220V ± 22V.
3. Is this unit truly portable?
Yes. The unit is relatively lightweight at about 18.8 kg and has a compact design intended for portability between examination rooms, veterinary clinics, and mobile use. Always follow safe manual handling practices and secure the unit during transport.
4. What applications is this machine intended for?
The MSLGX11 is optimized for orthopedic radiography and is intended for use in veterinary clinics and human medical settings where orthopedic imaging is required. Use in human medicine should follow local regulatory and facility requirements.
5. What safety certifications and classifications does it have?
The instrument is classified as Class I (non‑invasive diagnostics) and is CB certified for safety and quality assurance. Users must still follow radiation safety protocols and local regulatory requirements.
6. What are the power requirements and electrical specifications?
The unit operates from an AC power source with a rated input of AC220V ± 22V. Ensure your facility’s power supply matches this specification and that proper grounding is provided.
7. What warranty and after‑sales support are provided?
The product includes a 1‑year warranty. After‑sale service is provided via online technical support for troubleshooting and guidance. For repairs and parts replacement beyond online support, contact the distributor or manufacturer.
8. What is meant by 'shelf life: 1 year'?
The listing indicates a shelf life of 1 year, which generally refers to recommended storage timeframe or the period the manufacturer warrants storage stability. For installation and regular use, follow manufacturer guidelines and verify warranty/expiry details with your supplier.
9. Does the MSLGX11 include a digital detector (DR panel) and what file formats are supported?
The product description identifies the unit as a digital radiography solution but does not specify an included DR detector or supported file formats. Please confirm with the supplier whether a DR panel is included and which image formats (e.g., DICOM) are supported, or whether it is compatible with your existing detector.
10. What image quality can be expected for orthopedic examinations?
The high‑frequency generator and specified output parameters deliver clear, high‑contrast images suited to orthopedic diagnostic needs. Actual image quality will depend on detector performance, exposure parameters, positioning, and post‑processing.
11. What operator qualifications are required to use this X‑ray machine?
Operation should be performed by or under the supervision of trained and qualified radiography personnel in accordance with local laws and institutional policies. Proper radiation protection training is required for anyone operating or assisting with the equipment.
12. What routine maintenance is required?
Routine maintenance includes keeping the unit clean and dry, inspecting cables and connectors, verifying proper function of controls and displays, and following any manufacturer‑recommended calibration and preventive maintenance schedules. For service beyond basic checks, contact authorized service personnel.
13. Is professional installation and calibration required?
Yes. Proper installation, grounding, and calibration by qualified technicians are recommended to ensure safe operation and accurate imaging. Installation may include alignment, electrical hookup, and verification of exposure output.
14. Can the MSLGX11 be used for both veterinary and human medical imaging?
The unit is marketed for both medical and veterinary use. Use in human healthcare settings must comply with applicable medical device regulations, facility policies, and radiation safety laws. Confirm regulatory compliance and intended use with the manufacturer or distributor.
15. Who should I contact for compatibility questions, additional accessories, or replacement parts?
Contact the manufacturer or your authorized distributor for questions about detector compatibility, optional accessories (cables, stands, collimators, protective gear), spare parts, and service agreements. They can provide model‑specific recommendations and ordering information.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading