B21, China Town Mall, Midrand
311nm UVB Lamp Portable Physiotherapy Light for Atopic Dermatitis Vitiligo Psoriasis Skin Diseases Treatment
- Section : Medical Supplies
- Category : Physical Therapy Equipment
- SKU : 11000020518587

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 12 Feb, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the 311nm UVB Lamp and how does it work?
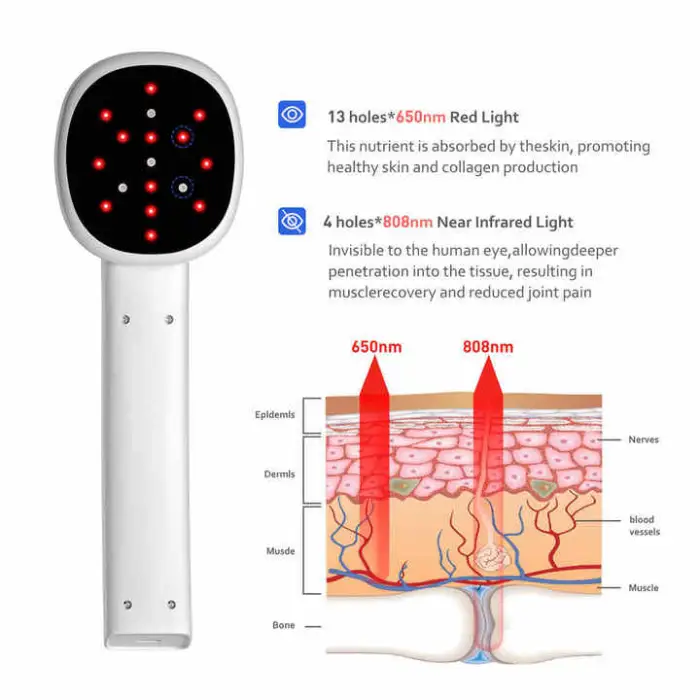
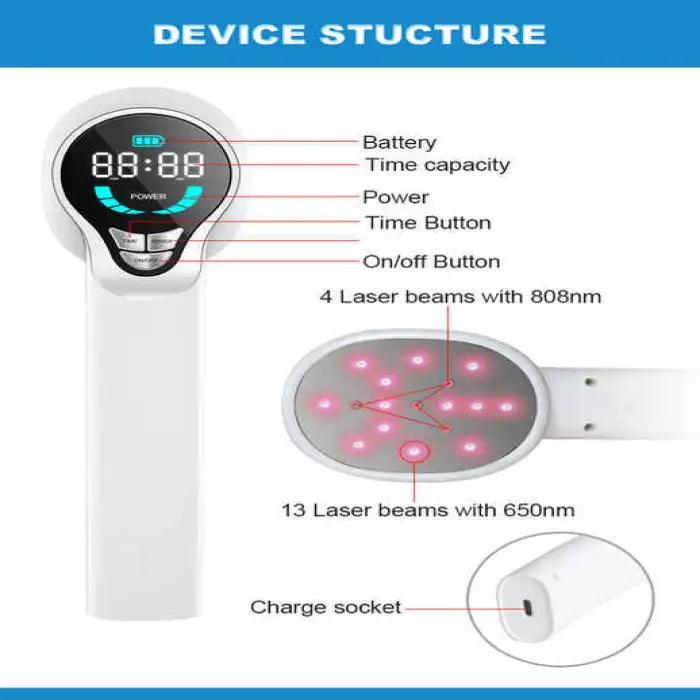
The device is a portable phototherapy light that emits narrowband UVB at 311nm, a wavelength commonly used in dermatology. This targeted UVB exposure can help reduce inflammation and modulate skin cell behavior in conditions such as atopic dermatitis, vitiligo and psoriasis. Use under medical guidance.
2. Which skin conditions is this lamp intended to help treat?
The lamp is intended for management of atopic dermatitis (eczema), vitiligo patches and psoriasis flare-ups. It is designed as an adjunctive phototherapy device to support skin health and recovery.
3. Is this device safe and certified?
The product is a Class II medical device (Model KJR-JGSG-B-1, Brand: LASTEK) and carries CE, RoHS, ISO13485 and ISO9001 certifications. Safety classification listed as Type II BOEM/ODMA. Always follow the user manual and a healthcare professional's instructions.
4. Can I use the lamp at home or while traveling?
Yes — the lamp is portable and designed for home and on‑the‑go use. However, phototherapy should be performed according to a treatment plan set by a dermatologist or qualified clinician.
5. Do I need protective eyewear or other precautions during use?
Yes. Always protect your eyes with appropriate UV-blocking goggles and avoid exposing non-target areas. Do not look directly at the light. Follow the safety and exposure instructions in the user manual and your clinician's guidance.
6. How often should I use the lamp and how long are sessions?
Treatment frequency and session duration vary by condition, skin type and clinical protocol. Typical regimens are individualized by a healthcare professional. Do not self-prescribe exposure times—consult your dermatologist for a safe treatment schedule.
7. What side effects or reactions might occur?
Possible side effects include temporary redness, itching, dry skin, or mild burns if overexposed. Long-term risks of UV exposure include photoaging and increased cancer risk; therefore use only under medical supervision. Stop use and seek medical attention if you experience severe reactions.
8. Can I use topical medications or other treatments at the same time?
Some topical agents and systemic medications are photosensitizing or may interact with UV therapy. Always consult your prescribing clinician before combining treatments. They may advise timing or temporary discontinuation of certain products.
9. Is the device suitable for children or pregnant people?
Use in children or during pregnancy should only occur under direct medical supervision. A dermatologist should evaluate risks and benefits before initiating phototherapy in these populations.
10. How do I clean and maintain the lamp?
Unplug/power off before cleaning. Wipe the housing and lamp cover with a soft, dry or lightly damp cloth and mild detergent if needed. Do not immerse the device in water or use abrasive cleaners. Refer to the user manual for specific maintenance instructions.
11. How long do the lamps/bulbs last and are replacements available?
Lamp life and replacement availability are not specified in the listing. Contact LASTEK or your seller for details on expected lamp lifetime, replacement parts and authorized service.
12. What should I do if the device malfunctions?
Stop using the device immediately. Refer to the troubleshooting section of the user manual and contact LASTEK customer support or your authorized distributor for repair, replacement or further instructions. Do not attempt unauthorized repairs.
13. Does the device require a specific power source or battery?
Power/battery details are not provided in the brief specification. Check the included user manual or contact the seller/manufacturer for exact power requirements and whether an adapter, battery or charger is included.
14. Are there any contraindications or people who should not use this lamp?
Contraindications can include a history of photosensitivity disorders, certain genetic conditions, active skin cancer, recent use of photosensitizing drugs, or other conditions specified by a clinician. Always consult your dermatologist before starting phototherapy.
15. Is there warranty or customer support information available?
Warranty and support details are not listed here. For warranty terms, after‑sales service and technical support, contact LASTEK or the seller from whom you purchase the device.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals