B21, China Town Mall, Midrand
Wonderfu HHL800a Fixer – Stainless Steel
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1600729150450

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 29 Mar, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
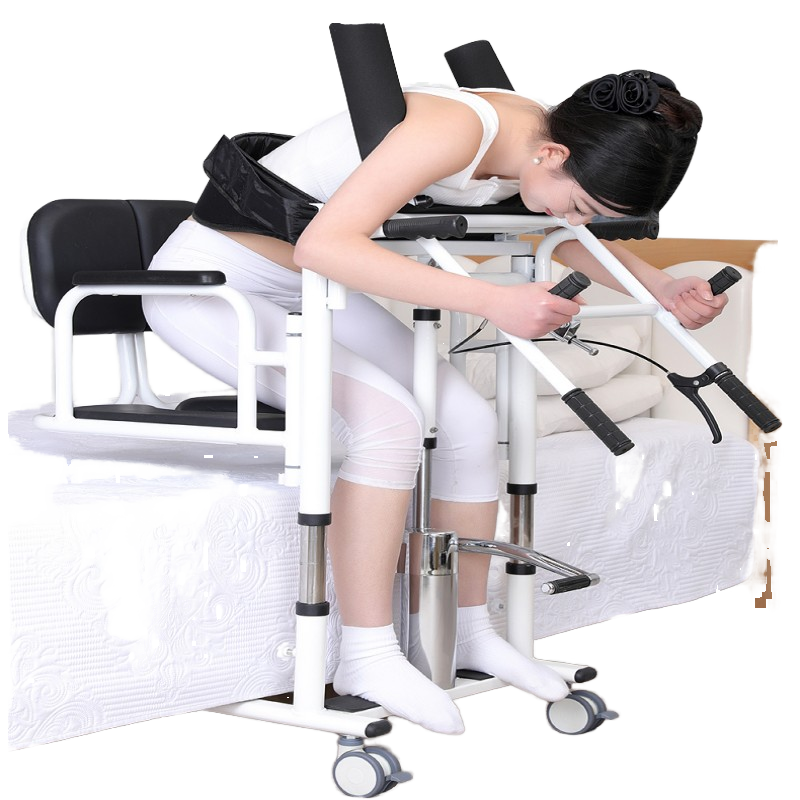
1. What is the Wonderfu HHL800a Fixer?
The Wonderfu HHL800a Fixer is a Class II medical instrument made from stainless steel, designed to provide reliable fixation in a variety of healthcare and rehabilitation settings. It is intended for use by medical professionals for therapeutic and procedural fixing applications.
2. What does 'Class II' mean for this device?
Class II indicates the device is subject to specific regulatory controls to ensure safety and effectiveness in medical use. It typically requires compliance with performance standards, quality systems and appropriate labeling; check local regulations for details.
3. What material is the HHL800a made of and why is that important?
The HHL800a is constructed from stainless steel, which offers enhanced strength, durability and resistance to corrosion. These properties make it suitable for repeated clinical use and common sterilization processes when used according to the manufacturer’s instructions.
4. What certifications does the Wonderfu HHL800a have?
The device is CE marked and manufactured under an ISO 13485 quality management system, indicating conformity with applicable European safety requirements and internationally recognized medical device quality standards.
5. What clinical or therapeutic applications is this fixer used for?
It is designed for a wide range of fixing and stabilization tasks in healthcare and rehabilitation environments, including clinical procedures, patient support and assistive/therapeutic applications. Specific uses depend on clinician judgment and facility protocols.
6. Is the HHL800a intended for implantation (internal fixation)?
No. The product description refers to fixation in clinical and rehabilitation settings and does not state it is implantable. For implantable use you must confirm with the manufacturer and applicable regulatory documentation; do not implant unless explicitly authorized by the manufacturer.
7. How should the device be sterilized?
Stainless steel instruments are commonly compatible with standard hospital sterilization methods (for example, steam autoclaving), but you must follow the manufacturer’s provided instructions for use (IFU) and your facility’s sterilization protocols to ensure safety and device integrity.
8. What are the recommended cleaning and maintenance procedures?
Clean the device promptly after use with compatible detergents and non‑abrasive brushes, rinse thoroughly, and follow validated disinfection/sterilization processes. Inspect regularly for wear, damage or corrosion and remove from service if defects are found. Always follow the manufacturer’s maintenance guidance.
9. How should the fixer be stored?
Store in a clean, dry environment away from corrosive chemicals and extremes of temperature or humidity. Protect from mechanical damage and keep in original packaging or dedicated instrument trays when not in use.
10. Is the HHL800a compatible with other devices or accessories?
The fixer is intended for general fixing applications; compatibility with specific accessories or systems depends on the accessory design. Verify compatibility with the accessory manufacturer or consult the Wonderfu IFU or technical support before combining devices.
11. Who is authorized to use the Wonderfu HHL800a?
The device should be used by trained healthcare professionals or personnel under their supervision, following institutional protocols and the manufacturer’s instructions for safe and effective use.
12. Are there any contraindications or safety precautions?
Follow standard clinical contraindications for fixation procedures and device‑specific warnings in the IFU. Use only for intended indications, ensure proper patient assessment, and inspect the device before use. Do not use if damaged or corroded.
13. What warranty and service options are available?
Warranty and service terms are not provided in the basic product description. Contact the manufacturer or your authorized supplier for details on warranty coverage, service plans, repairs and spare parts.
14. What is the expected lifespan of the stainless steel fixer?
While stainless steel offers long-term durability, actual lifespan depends on frequency of use, maintenance, sterilization cycles and handling. Proper cleaning, inspection and storage will extend service life; refer to the manufacturer’s recommendations for end‑of‑life criteria.
15. How should I dispose of the device when it is no longer usable?
Dispose of medical devices in accordance with local laws and healthcare facility policies. Where possible recycle stainless steel components through appropriate metal recycling channels, and follow any regulations for disposal of medical equipment and contaminated instruments.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading