B21, China Town Mall, Midrand
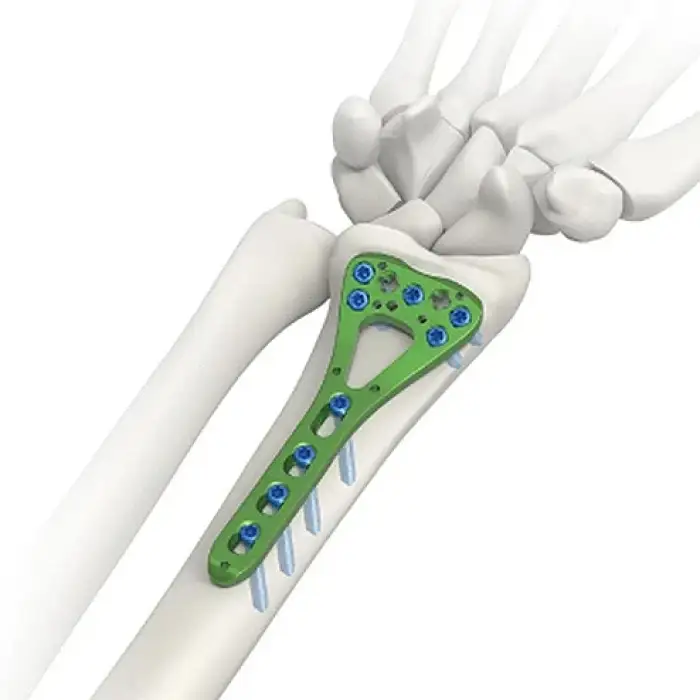
Trauma Fracture Fixation Titanium Implants by Jinlu
- Section : Medical Supplies
- Category : Bone Surgical Instruments
- SKU : 60686648686-1720610925

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 30 Jan, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What material are the Trauma Fracture Fixation Titanium Implants made from?
The implants are made from premium titanium, which offers strength, corrosion resistance, and biocompatibility.
2. What classification do these implants fall under?
These trauma fracture fixation implants are classified as Class III medical devices, ensuring they meet stringent safety and performance standards.
3. Are the implants compliant with international standards?
Yes, the implants are CE, ISO13485, and GMP certified, ensuring compliance with international medical device regulations for safety and quality.
4. What types of fractures are these implants designed for?
These implants are specifically designed for the fixation of trauma fractures, providing stability during the healing process.
5. Can the implants be customized for specific surgical needs?
Yes, OEM services are available for customization, allowing tailored solutions to meet specific surgical and patient needs.
6. What color options are available for the implants?
The implants are available in grey, or can be customized to other colors upon request to match surgical preferences.
7. How are the implants shipped?
The implants are shipped through reliable services such as DHL, TNT, FedEx, Aramex, UPS, and EMS, ensuring safe and timely delivery from Shanghai, China.
8. What is the significance of the AO orthopedic implant standard?
The implants conform to the AO orthopedic implant standards, which provides optimal fixation and support for fracture healing.
9. What is the weight advantage of titanium implants?
Titanium implants are lightweight compared to other materials, making them easier to handle and implant during orthopedic surgeries.
10. Are there different types of locking screws used with the implants?
Yes, the implants use various locking screws, such as φ2.7 locking screws and φ2.5 cortical self-tapping screws, depending on the specific implant model.
11. How can surgeons ensure compatibility with these implants?
Surgeons can ensure compatibility by reviewing the specifications provided for each implant type, which detail the required screws and application methods.
12. What benefits do titanium implants provide for patients?
Titanium implants provide durability, resistance to corrosion, and biocompatibility, which contribute to long-lasting stability and support during recovery.
13. What should be considered when selecting an implant for a patient?
Considerations include the type of fracture, the patient's overall health, and the specific requirements of the surgical procedure.
14. Is there a risk of rejection with titanium implants?
Titanium is biocompatible, which means it is generally well accepted by the body, making rejection very rare.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals