B21, China Town Mall, Midrand
SW-2100AB Low Ophthalmic Instrument Optical Eye Sight Examination A/B Scanning Machines
- Section : Consumer Electronics
- Category : Testing Equipment
- SKU : 10000015170559

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 30 Mar, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
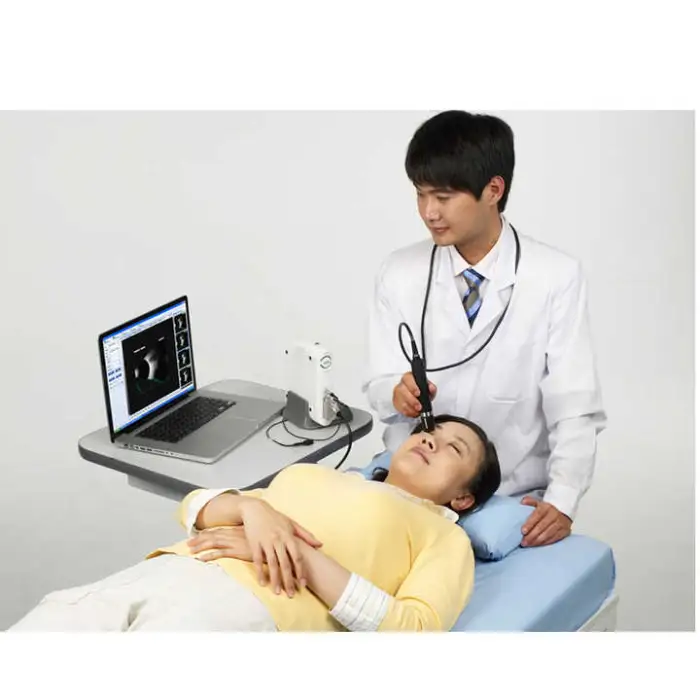
1. What is the SW-2100AB Low Ophthalmic Instrument?
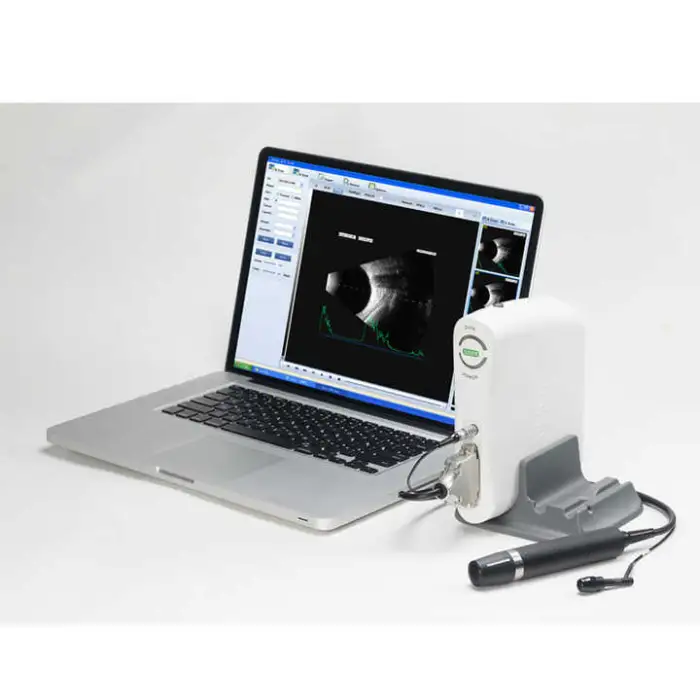
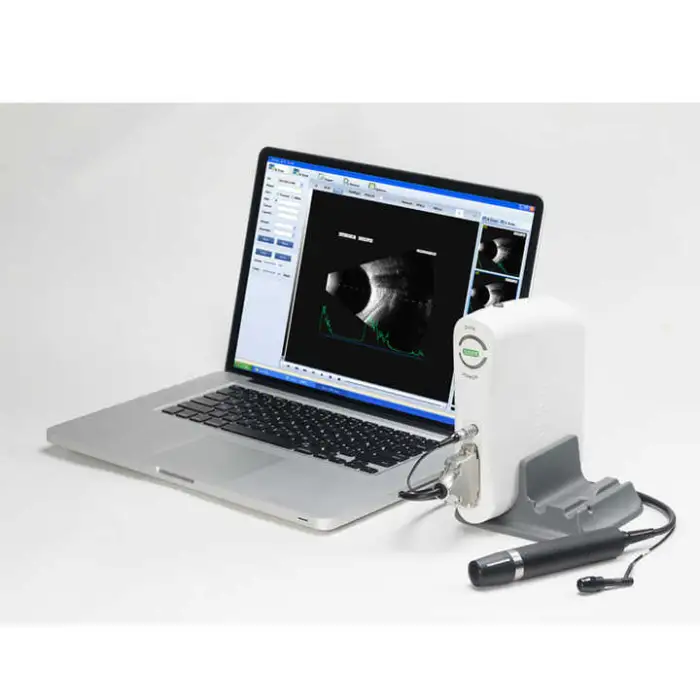
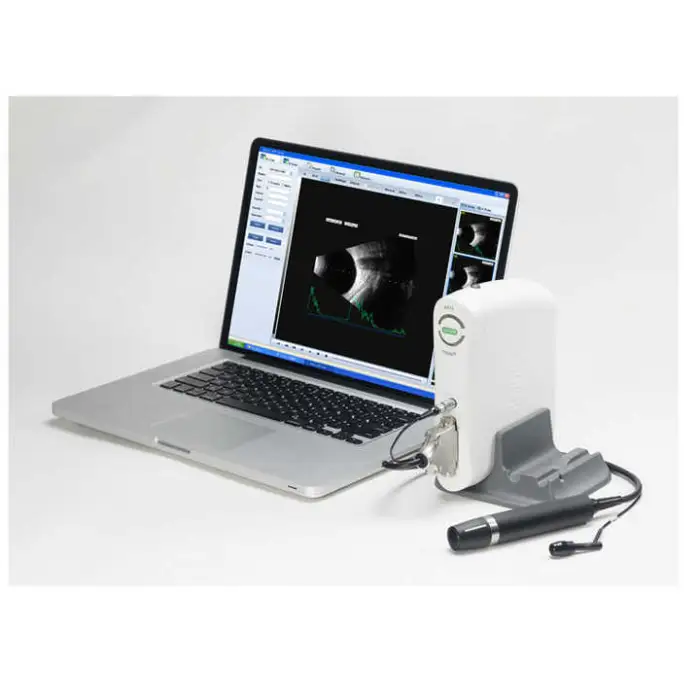
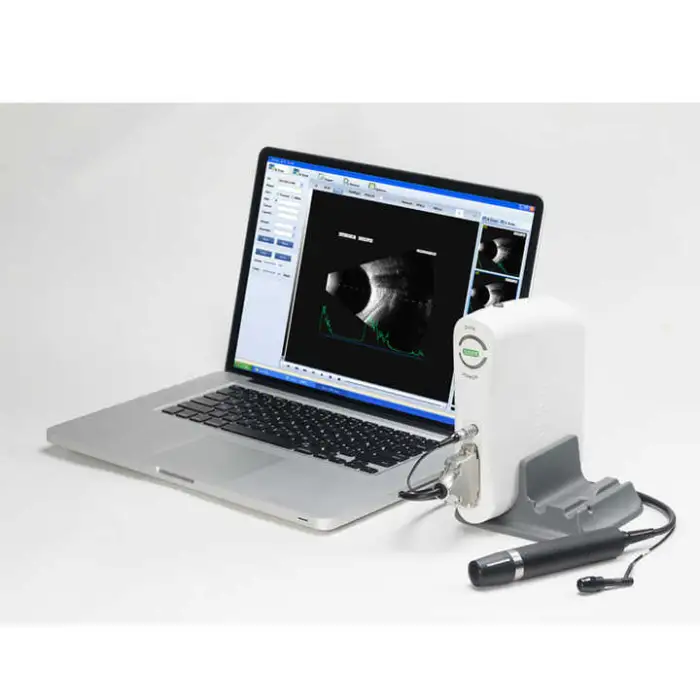
The SW-2100AB is an ophthalmic A/B scan ultrasound instrument designed for eye examinations. It provides high-quality A/B scanning images to help clinicians assess ocular structure, assist in diagnosis, and support pre-operative evaluations and routine eye check-ups.
2. What are the key scanning specifications of the device?
The device uses A/B scanning technology with a B-scan frequency of 10 MHz. It performs sector scanning and offers multi continuous and real-time magnification for detailed imaging.
3. What is the imaging resolution and positional precision?
Resolution is specified as lateral ≤ 0.3 mm and vertical ≤ 0.2 mm. Geometry position precision is lateral ≤ 5% and vertical ≤ 3%.
4. How deep can the SW-2100AB image?
The maximum imaging depth of the SW-2100AB is 60 mm.
5. Is the operation noisy?
No — the B-scan is magnetic driven and described as noiseless for a more comfortable clinical environment.
6. What clinical applications is this instrument suited for?
Typical uses include general vision assessments in eye clinics, diagnosing intraocular conditions, pre-operative eye evaluations, routine ophthalmic check-ups, and supporting ophthalmology research and studies.
7. What is the model number and packaging?
The model number is MSLYZ09. The unit is shipped in a standard export package.
8. How many units can you supply and how do I place an order?
The product listing indicates a supply ability described as '1000 amperes per Month' — please contact the supplier or sales representative to confirm monthly production capacity, current stock, lead times, pricing, and to place an order.
9. What accessories, probes, or software are included or compatible?
The product description does not list included accessories or compatible probes/software. Contact the manufacturer or distributor for a complete list of supplied accessories and supported probes, cables, mounts, and software options.
10. What are the power requirements and installation needs?
Power requirements and installation details are not provided in the summary. Request the technical datasheet or installation manual from the supplier to confirm electrical specifications, mounting requirements, and recommended clinical setup.
11. What maintenance, calibration, and cleaning are recommended?
Routine maintenance and calibration intervals are not listed here. Follow the manufacturer's user manual for cleaning instructions, scheduled calibration, and preventive maintenance. Arrange service and calibration with authorized technicians to ensure accurate performance.
12. Is training provided for clinical staff?
The device is described as compact and easy to use, but specific training provisions are not stated. Ask your supplier about on-site or remote training, user guides, and clinical support options when purchasing.
13. Does the SW-2100AB have regulatory approvals (e.g., CE, FDA)?
Regulatory clearances are not mentioned in the provided description. Verify applicable certifications and regulatory approvals (CE, FDA, or local health authority clearance) with the manufacturer or distributor before clinical use.
14. What warranty and after-sales support are available?
Warranty and after-sales support details are not included in the product summary. Contact the seller or manufacturer to obtain warranty terms, service contracts, spare parts availability, and technical support options.
15. How can I get more technical documentation or request a demo?
For full technical specifications, user manuals, clinical performance data, or to arrange a demonstration, contact the manufacturer or authorized distributor directly. They can provide datasheets, training information, and demo scheduling.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading