B21, China Town Mall, Midrand
NMES EMG Biofeedback FES Body Nerve Muscle Stimulator Clinic Use Physical Therapy Equipment for Health Care & Stroke Recovery
- Section : Medical Supplies
- Category : Household Medical Devices
- SKU : 1601449889820

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 30 Mar, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
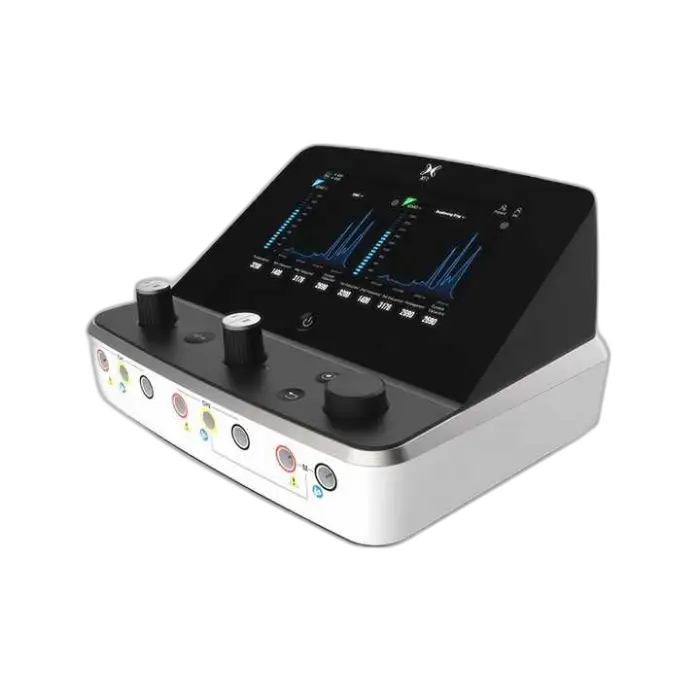
1. What is the NMES EMG Biofeedback FES Body Nerve Muscle Stimulator (Model XFT-2003K)?
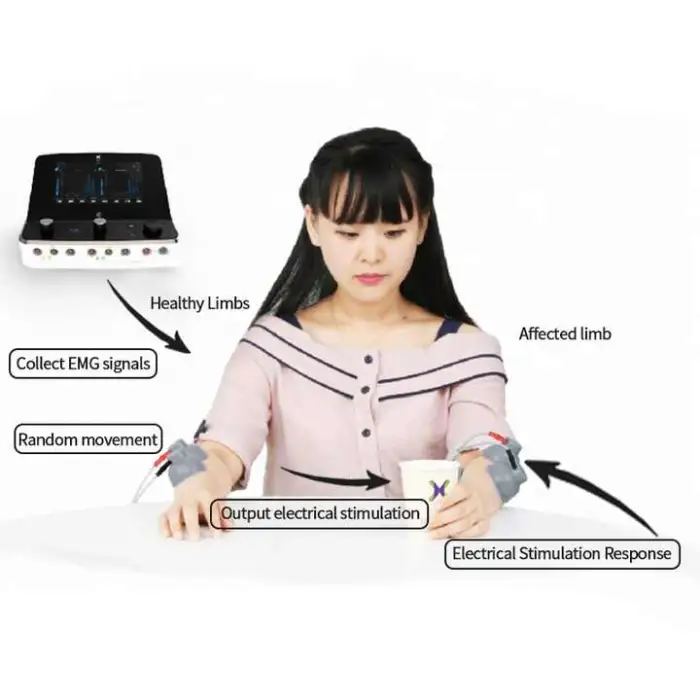
It is a multi-modal physical therapy device combining NMES (neuromuscular electrical stimulation), FES (functional electrical stimulation) and EMG biofeedback to help restore muscle activation, improve strength and support rehabilitation (commonly used in stroke recovery and other neuromuscular conditions).
2. Who is this device intended for?
It is intended for use by clinicians and trained caregivers in hospitals, clinics and rehabilitation centers, and can also be used at home by patients under the guidance of a healthcare professional for conditions such as post-stroke weakness, muscle atrophy and certain nerve or muscle disorders.
3. How do NMES, FES and EMG biofeedback work?
NMES/FES deliver controlled electrical pulses to motor nerves to produce muscle contractions and facilitate strength/retraining. EMG biofeedback monitors voluntary muscle activity and provides feedback to help patients learn to activate muscles more effectively. Combined, they promote motor relearning and functional recovery.
4. Is the device safe to use?
When used according to the manufacturer’s instructions and under appropriate supervision, the device is generally safe. However, electrical stimulation has contraindications and precautions—consult a healthcare professional before use. Do not use if you have a pacemaker or implanted electronic device, pregnant abdomen/chest, uncontrolled epilepsy, malignant tumor, or over broken skin or areas of poor sensation unless directed by a clinician.
5. Can I use this device at home?
Yes — the unit is portable and rechargeable for home use, but only after assessment and instruction from a qualified clinician. Individual treatment parameters, electrode placement and safety checks should be established by a therapist or physician.
6. How do I start a treatment session?
Basic steps: charge the device; prepare and clean the skin; attach electrodes to the treatment area per clinician guidance; power on the unit; select the appropriate program/mode and intensity; monitor patient comfort and response throughout the session. Follow the user manual and clinician's protocol.
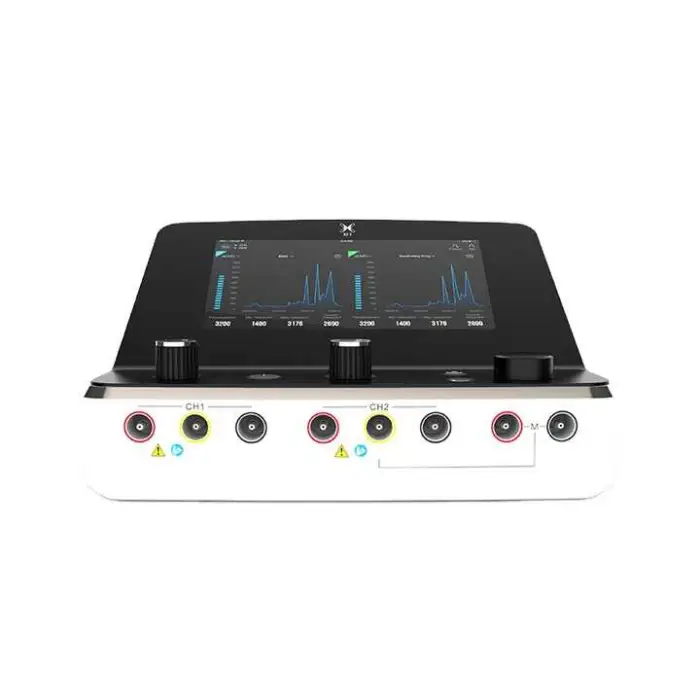
7. Where should electrodes be placed?
Electrode placement depends on the targeted muscle or nerve and should be determined by a trained clinician. Generally, place electrodes over the motor points or along the muscle belly, avoid the throat/carotid sinus, eyes, mouth, or over broken skin. Always follow the included placement diagrams or clinical guidance.
8. How long and how often should treatments be?
Typical sessions range from 10 to 30 minutes depending on the therapy goals and clinical protocol; frequency may be daily or several times per week. Your therapist will prescribe session length and frequency tailored to the patient's condition and tolerance.
9. What are the power and battery specifications?
The device uses a rechargeable lithium battery and accepts AC 100–240V, 50–60Hz, 60VA for charging. Actual runtime per charge depends on program intensity and usage; refer to the product manual or vendor listing for exact charging time and expected battery life.
10. What consumables and accessories will I need?
Common consumables include reusable or disposable electrode pads, lead wires, and skin-prep supplies (alcohol wipes, conductive gel if needed). Electrode pads wear out with use and must be replaced periodically. Check the product package or vendor listing to confirm which accessories are included.
11. How should I clean and maintain the device?
Turn off and disconnect electrodes before cleaning. Wipe the device housing with a soft cloth slightly dampened with a mild disinfectant or 70% isopropyl alcohol; do not immerse in liquid. Clean skin and electrodes per manufacturer instructions and store the unit and accessories in a dry, cool place. Replace damaged leads or pads.
12. Are there any common troubleshooting tips?
If stimulation feels weak or intermittent: check electrode adhesion and skin contact, clean or replace pads, ensure lead wires are connected firmly, and confirm battery is charged. If the device will not power on, try charging it and verify the AC adapter and outlet. Contact vendor support for persistent faults.
13. Do I need training to operate the device?
Yes. Although the unit is designed to be user-friendly, appropriate training from a clinician or vendor representative is recommended to set safe parameters, correct electrode placement and interpret EMG biofeedback data.
14. What regulatory approvals or warranty does the product have?
Regulatory approvals (for example CE or FDA clearance) and warranty details are not listed here. Check the seller’s product page, user manual or contact the vendor directly for information about certifications, safety testing and warranty terms.
15. Can this device be used with other electrical medical equipment?
Exercise caution when using multiple electrical medical devices simultaneously. Avoid placing electrodes over other electronic implants and do not operate near sensitive medical equipment without professional guidance. Always follow facility policies and the device manual regarding electromagnetic compatibility.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading