B21, China Town Mall, Midrand
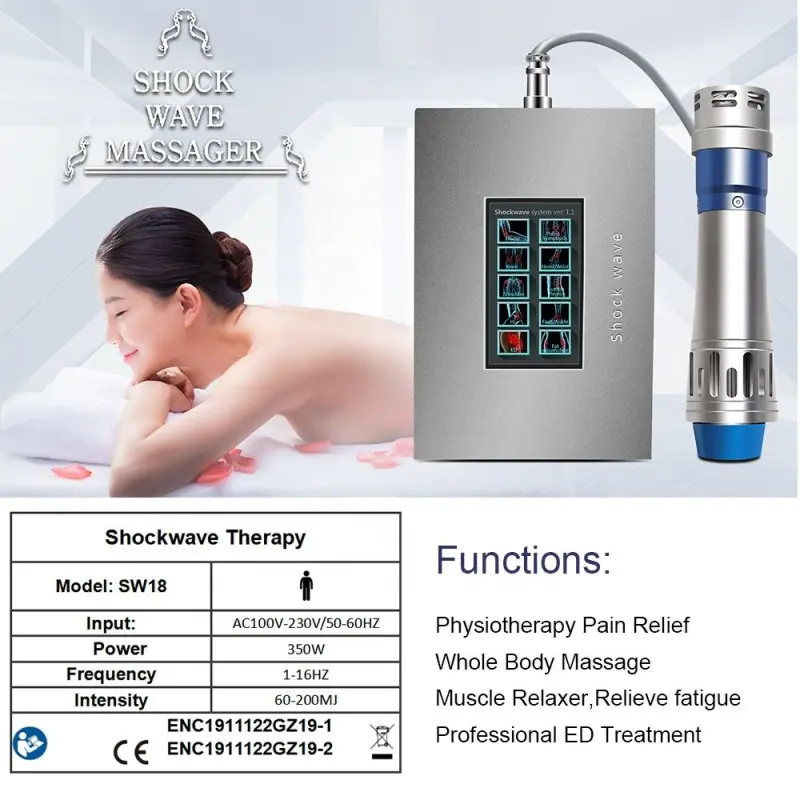
Huanshi G-SW18 Shock Wave Physical Therapy Medical Equipment
- Section : Medical Supplies
- Category : Physical Therapy Equipment
- SKU : 62421905013

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 11 Mar, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Huanshi G-SW18 Shock Wave Physical Therapy Medical Equipment?
The G-SW18 is a Class I radial shockwave therapy (RSWT) device for clinics and medical settings. It delivers noninvasive acoustic shockwaves to reduce pain and promote healing in musculoskeletal conditions used in physical therapy, rehabilitation, and pain management.
2. How does radial shockwave therapy (RSWT) work?
RSWT uses pulsed acoustic waves applied to damaged tissue to stimulate local blood circulation, reduce pain, and promote collagen production and tissue regeneration. Treatment is delivered through a handpiece and targeted transmitters.
3. Which conditions can the G-SW18 treat?
Common indications include tendonitis (shoulder, elbow), plantar fasciitis, heel spurs, Achilles tendinopathy, patellar tendinitis, myofascial trigger points, trochanteric bursitis, sports injuries, some post-muscle injury conditions and selected aesthetic uses (cellulite, skin irregularities).
4. Can the G-SW18 be used for erectile dysfunction (ED)?
The device supports extracorporeal shockwave therapy protocols used for some cases of ED (EDSWT) aimed at improving penile blood flow. Use for ED should follow established clinical protocols and be performed by trained medical professionals; results vary by patient.
5. Who should operate this device?
The G-SW18 should be operated by licensed healthcare professionals or trained clinicians experienced in shockwave therapy. Proper training in indications, contraindications and device settings is recommended.
6. What operating modes and adjustable settings does it offer?
The unit provides manual and auto modes for flexible treatment delivery. Key adjustable parameters include 12 intensity levels and a shot frequency range of 1–16 Hz to tailor energy and treatment speed per patient and indication.
7. What are the main technical specifications?
Typical specifications: voltage 110–240 V, frequency 50/60 Hz, maximum power ~90 W, maximum current ~2 A, intensity with 12 levels, shot frequency 1–16 Hz. Verify exact specs on the supplied product datasheet.
8. What is included in the package?
Standard package typically contains: 1 host unit, 1 shockwave work head, 7 transmitters and 7 covers, 1 power supply line, fittings, and a user manual. Confirm contents with your supplier.
9. What are contraindications and safety precautions?
Common contraindications include pregnancy, active infection or open wounds at the treatment site, malignant lesions in the treatment area, untreated blood clotting disorders or anticoagulant therapy, and treatment over growth plates. Avoid placing the probe over the heart, lungs or implants like pacemakers without specialist advice. Always consult the user manual and clinical guidelines.
10. What cleaning and maintenance are required?
Clean the exterior with a soft, damp cloth and approved disinfectant. Disinfect work heads and covers between patients according to local infection control policies. Do not immerse the device in liquid. Inspect cables and connectors regularly; follow the manual for recommended maintenance and service intervals.
11. Is clinical evidence available supporting effectiveness?
Radial shockwave therapy has been studied for conditions like plantar fasciitis and lateral epicondylitis and is supported by clinical literature showing pain reduction and functional improvement for many patients. Individual outcomes vary; treatments should follow evidence-based protocols.
12. What warranty and service options are available?
The G-SW18 comes with a one-year warranty. ODM and OEM services are available for customization. For repairs, calibration or extended service plans, contact the manufacturer or authorized distributor.
13. Are there training or certification recommendations?
Yes. Operators should receive device-specific training covering indications, contraindications, parameter selection and hands-on use. Many regions require licensed clinicians to perform shockwave therapy; follow local regulations and professional guidelines.
14. What safety features does the device have?
Safety features include controlled adjustable energy levels, built-in operating modes (manual/auto), and protective transmitters/covers. The targeted application minimizes stress to surrounding tissues compared with more invasive options. Always follow operating instructions to avoid misuse.
15. What are the package dimensions and shipping weight?
Typical package dimensions are about 38 x 30 x 13 cm. Shipping weight varies by model and accessories, generally in the range of approximately 3–5 kg. Confirm exact values with the seller before purchase.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading