B21, China Town Mall, Midrand
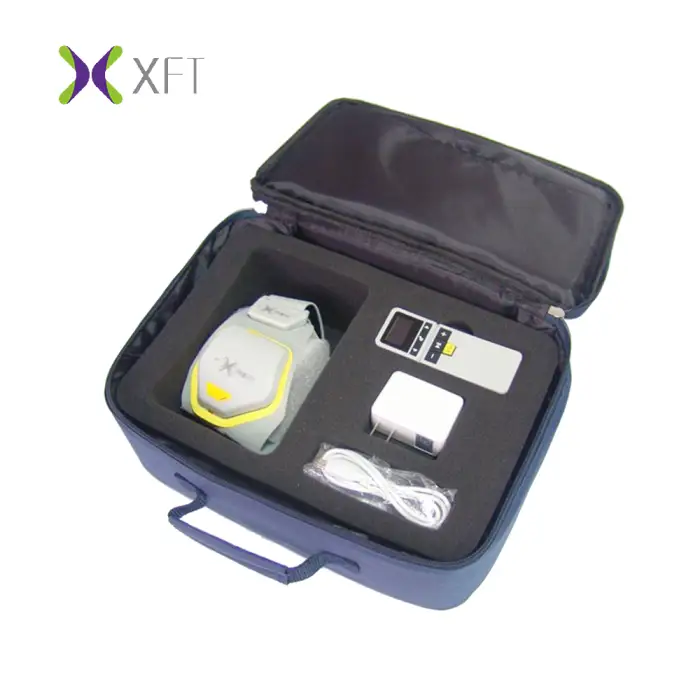
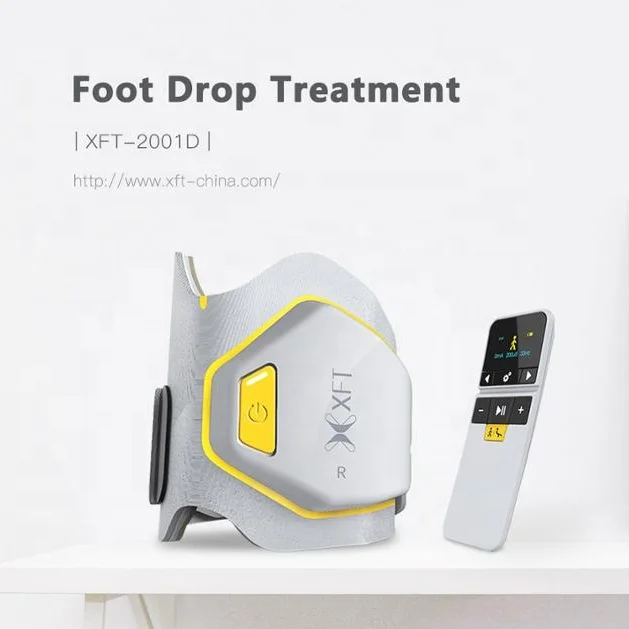
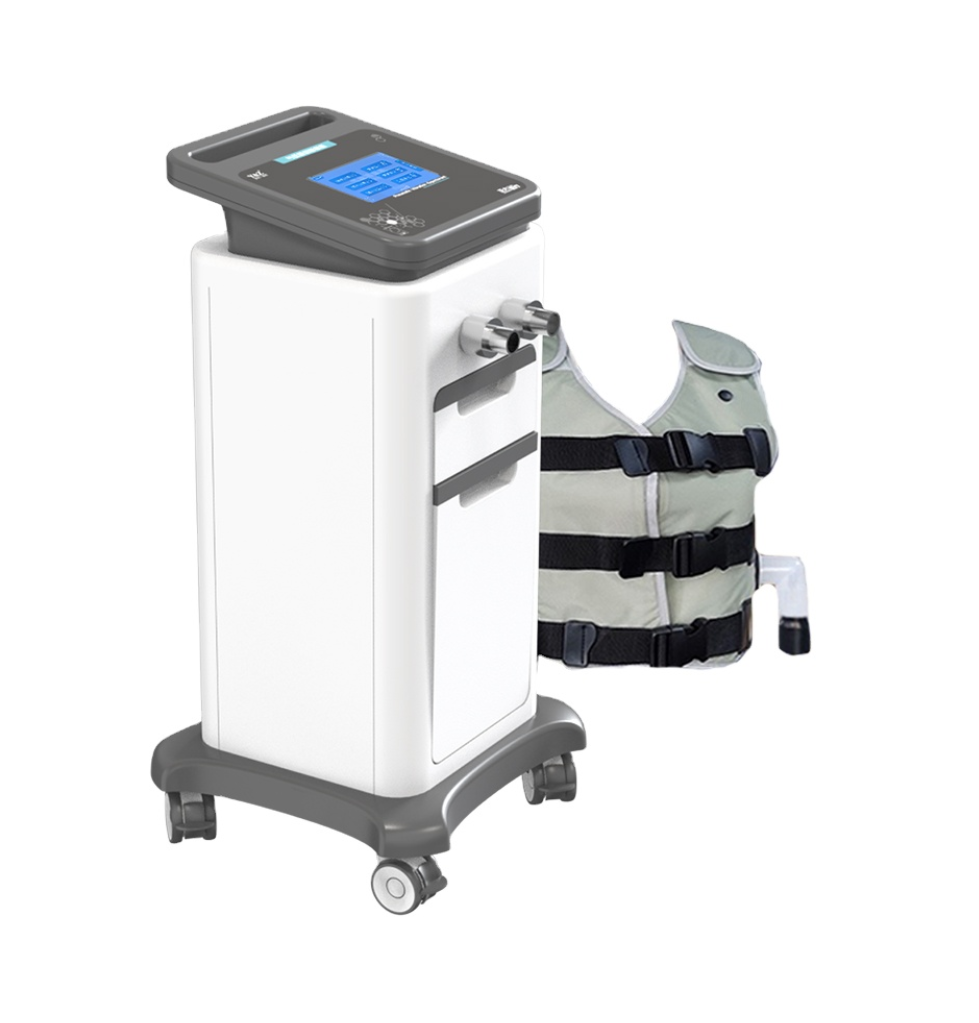
Foot Drop System XFT-2001D – Lower Limb Training Exercise
- Section : Medical Supplies
- Category : Rehabilitation Equipment
- SKU : 62318858844

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 17 Feb, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Foot Drop System XFT-2001D and who is it intended for?
The XFT-2001D is a Class II orthotic trainer designed to help people with foot drop caused by neurological conditions or nerve injury. It is intended for use in rehabilitation settings and at home under clinical guidance to improve lower limb movement and mobility.
2. How does the XFT-2001D work?
The device uses functional electrical stimulation (FES) guided by advanced MEMS sensors and intelligent algorithms to detect leg swing angle and pace, then precisely time and control stimulation to assist dorsiflexion during gait.
3. Which medical conditions can the XFT-2001D help with?
It is commonly used for foot drop related to stroke (CVA), multiple sclerosis (MS), spinal cord injury (SCI), traumatic brain injury (TBI), brain tumor, cerebral palsy (CP) and other brain or spinal disorders that affect ankle dorsiflexion.
4. Is the XFT-2001D a certified medical device?
Yes. The XFT-2001D is classified as a Class II medical device and carries CE marking and ISO 13485 certification, indicating it meets applicable regulatory and quality management standards.
5. What are the size and weight of the device?
The device is compact and lightweight with dimensions of 111 x 62 x 22 mm and a net weight of approximately 63 g, making it suitable for both clinical and home use.
6. What power source does the XFT-2001D use and what about battery life?
The XFT-2001D is battery powered. Specific battery type and expected runtime are provided in the product user manual. Batteries are not covered under the standard 5-year warranty.
7. How do I set up and start using the XFT-2001D?
Basic setup typically includes attaching the device and electrodes/straps per the user manual, configuring stimulation settings, and performing an initial calibration while standing or walking. Setup and parameter tuning should be performed by or under the supervision of a trained clinician or therapist.
8. Do I need a clinician to prescribe or fit the device?
Yes. Clinical assessment, prescription, and fitting by a qualified rehabilitation professional are recommended to ensure appropriate parameters, safe use, and optimal therapeutic outcomes.
9. Are there contraindications or precautions I should be aware of?
Common precautions include implanted electronic devices (e.g., pacemakers), pregnancy, active infection or open wounds at the electrode site, uncontrolled epilepsy, or severe sensory deficits. Always consult a clinician and refer to the device safety manual for complete contraindications and warnings.
10. Can the XFT-2001D be used in home environments?
Yes. The unit's compact, lightweight design makes it suitable for home use following clinician instruction, training, and ongoing follow-up to monitor progress and adjust settings as needed.
11. How should I clean and maintain the device?
Keep the device dry and clean by wiping with a soft, damp cloth and mild disinfectant as described in the manual. Inspect leads, electrodes, straps and connectors regularly and replace consumables (electrodes, batteries) per manufacturer guidance.
12. What does the 5-year warranty cover?
The product includes a 5-year warranty covering manufacturing defects and device malfunction under normal use. Consumables such as batteries and electrodes are typically excluded. Check the warranty terms provided by the supplier for full details and registration requirements.
13. Can the XFT-2001D be used together with ankle-foot orthoses (AFOs) or different footwear?
The device's compact design allows it to be used with many AFOs and types of footwear, but compatibility depends on the specific AFO and shoe. A clinician should evaluate fit and function to ensure the device does not interfere with orthosis performance.
14. Is there clinical evidence supporting the XFT-2001D's approach?
The XFT-2001D applies established FES principles and sensor-controlled stimulation commonly used in lower-limb rehabilitation. For device-specific clinical data, performance studies, or published research, consult the manufacturer's documentation or contact their clinical support team.
15. How do I purchase the XFT-2001D or get technical support and training?
Contact the manufacturer's authorized distributor or supplier to place an order, arrange demonstrations, request clinician training, or obtain spare parts and technical support. The supplier can provide information about local service, warranty registration and user training options.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading