B21, China Town Mall, Midrand
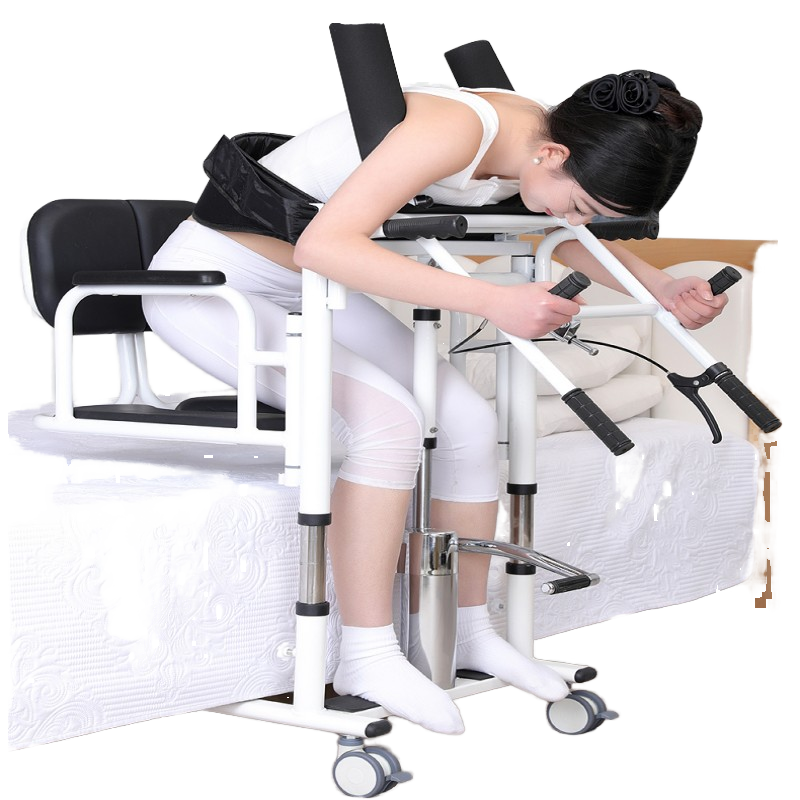
Foot Ankle Care Equipment Gait Analysis Sensor Platform Foot Rehabilitation Device
- Section : Medical Supplies
- Category : Rehabilitation Equipment
- SKU : 1601088856516

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 30 Mar, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Foot Ankle Care Equipment Gait Analysis Sensor Platform used for?
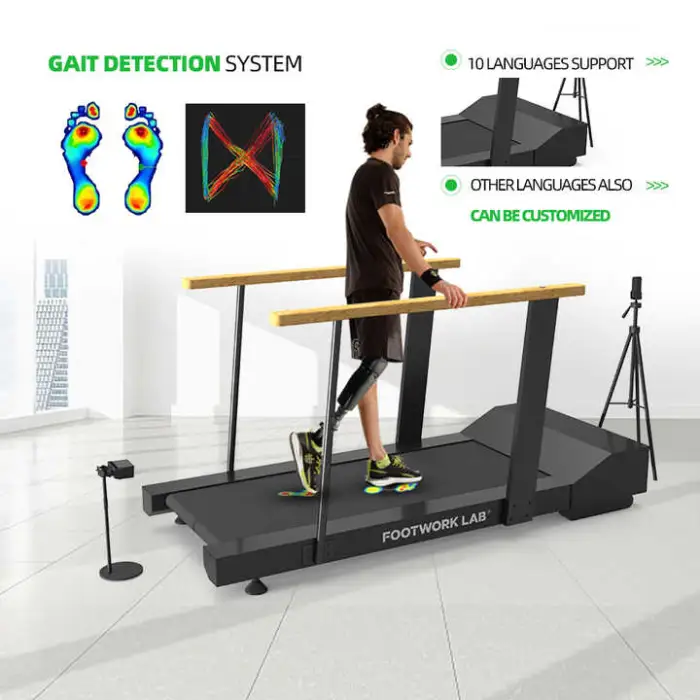
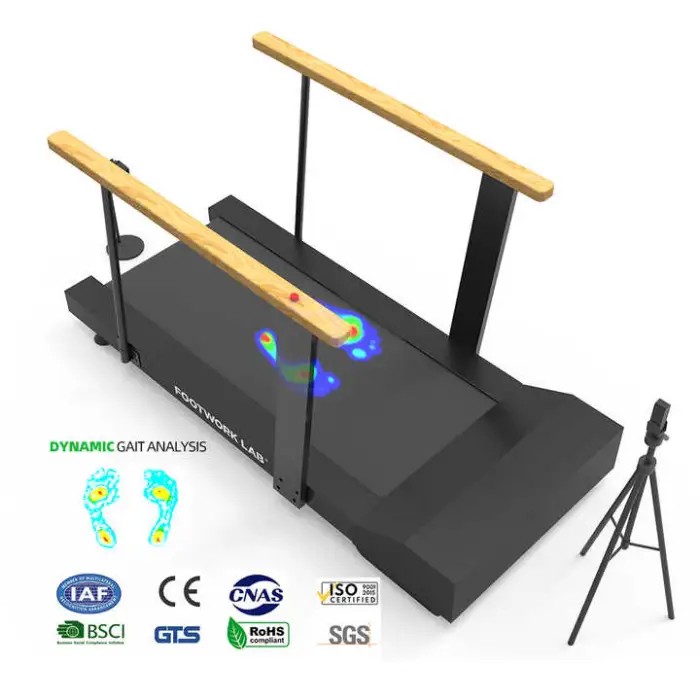
It is a dynamic gait analysis platform designed to assess and help improve walking patterns. Typical uses include foot and ankle rehabilitation, monitoring post-surgical recovery, athletic gait assessment, physical therapy support, and research/education in gait analysis.
2. What are the key features of this gait analysis device?
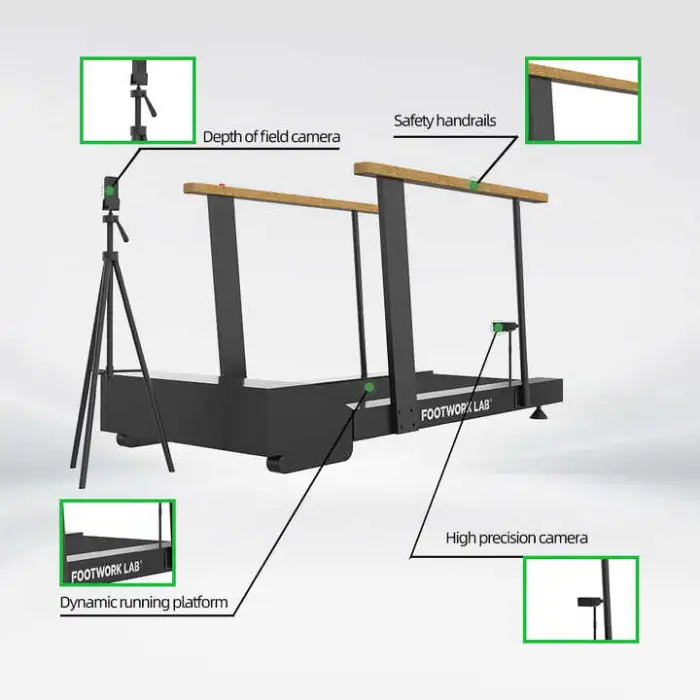
Key features include dynamic gait analysis for precise data, a user-friendly USB interface for connectivity, high optical resolution (1080) for clear imaging, durable construction, and availability in multiple colors (Gray, Silver, and others).
3. What are the physical dimensions and weight of the product?
The single package size is 101 x 51 x 181 cm, and the single gross weight is 155 kg. It is a substantial device intended primarily for clinical or research settings.
4. Does the platform come with an SDK for custom software integration?
No. According to the specifications, there is no Software Development Kit (SDK) available.
5. How does the device connect to a computer or other systems?
The device features a user-friendly USB interface for connectivity to a PC or workstation. For details about supported software and file formats, contact the vendor or check the product documentation.
6. What does 'optical resolution 1080' mean?
The listed optical resolution of 1080 indicates the device provides high-definition imaging (comparable to 1080p). For exact sensor specifications and how that resolution is used in measurements, consult the manufacturer or product manual.
7. Is this device suitable for home use?
Due to its size and weight (155 kg) and its design as a dynamic gait analysis platform, it is best suited for clinics, hospitals, rehabilitation centers, and research labs rather than typical home use.
8. Are spare parts or warranty support included?
The product specification notes free spare parts for 12 months. For full warranty terms, service agreements, and extended support options, contact the seller or manufacturer.
9. Does the device require regular calibration or maintenance?
Periodic calibration and maintenance are generally recommended for gait analysis equipment to ensure measurement accuracy. Follow the product manual for maintenance schedules and contact vendor support for calibration services.
10. What parameters can the platform measure (e.g., pressure, stride length)?
The platform is designed for dynamic gait analysis and provides precise gait data. Specific measured parameters (such as plantar pressure, center of pressure, stride length, cadence) depend on the device configuration and bundled software. Confirm the exact measurement outputs with the vendor.
11. Is the product compatible with electronic medical records (EMR) or third-party analysis tools?
Compatibility with EMR systems or third-party tools depends on the provided software and data export options. Since there is no SDK, integration capabilities may be limited—ask the vendor for details about supported file formats and integration options.
12. What operating environments and power requirements are needed?
The specification sheet does not list power or environmental requirements. Refer to the product manual or contact the seller for the device's power specifications, ambient temperature/humidity ranges, and installation requirements.
13. Is training required to operate the device?
While the device has a user-friendly interface, proper use for clinical assessment and data interpretation typically requires training. Vendors often provide training or documentation—check with the supplier for available training programs or user guides.
14. How should the platform be cleaned and maintained?
Use a soft cloth and mild cleaning agents as recommended by the manufacturer. Avoid harsh chemicals or abrasive materials. Follow the cleaning and maintenance instructions in the product manual to ensure safety and longevity.
15. How can I get more detailed specifications or request a demo?
For full technical specifications, software demonstrations, pricing, or to arrange a hands-on demo, contact the product seller or manufacturer directly. They can provide detailed datasheets, usability demonstrations, and answers tailored to your clinical or research needs.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading