B21, China Town Mall, Midrand
Cervical and Lumbar Traction Device and Spine Traction Bed and Orthopedic Traction Table
- Section : Medical Supplies
- Category : Rehabilitation Equipment
- SKU : 1600919674831

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 31 Mar, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
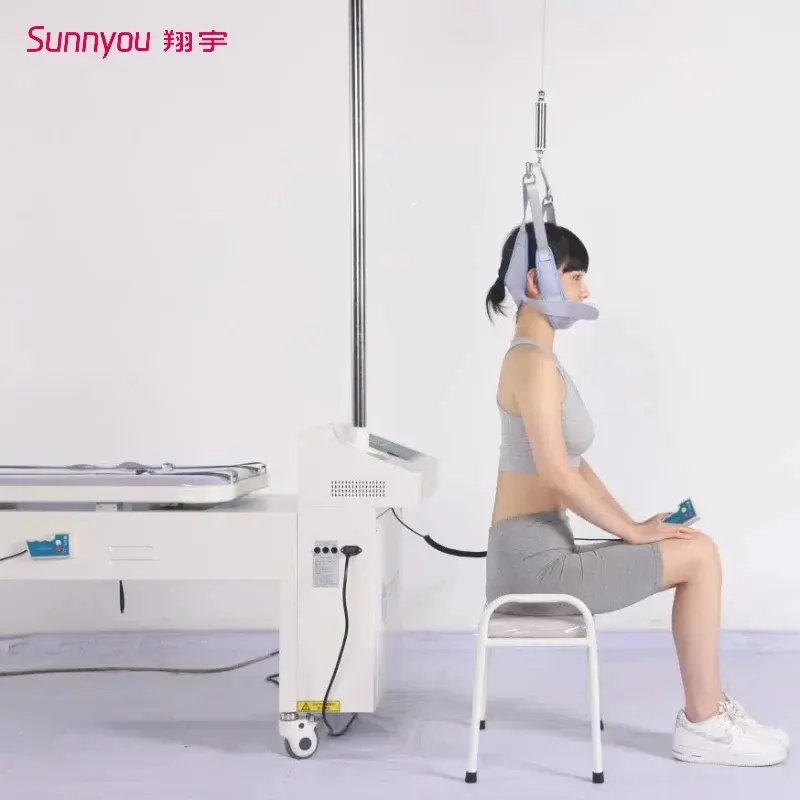
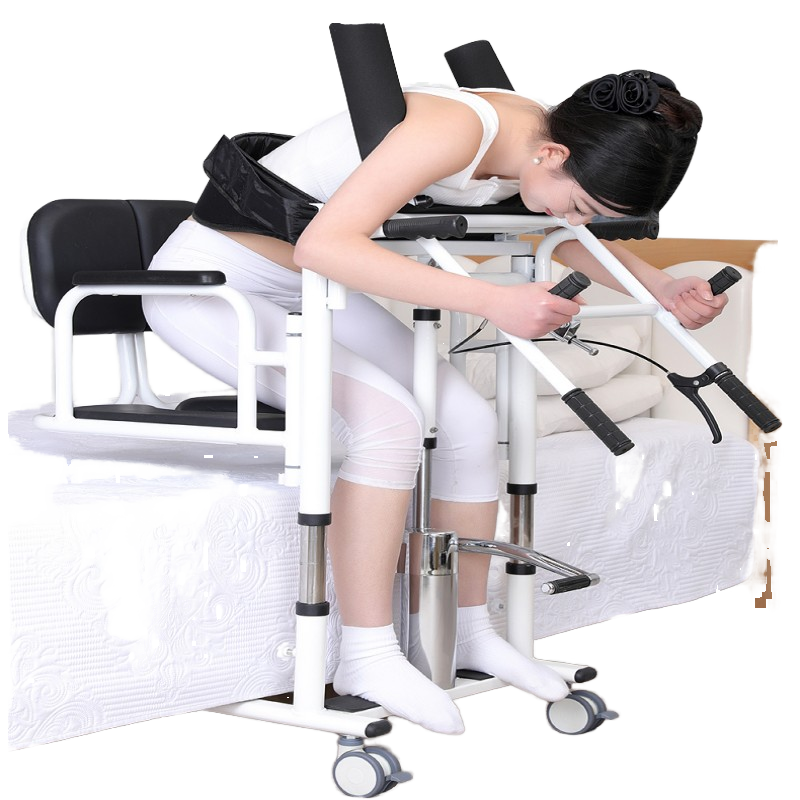
1. What is the Cervical and Lumbar Traction Bed (JYZ-IIB)?
The JYZ-IIB is a microcomputer-controlled, three-dimensional multifunctional traction bed designed for integrated cervical and lumbar traction. It uses a telescopic motor for traction drive and offers multiple automated traction programs for clinical use.
2. Which conditions is this traction bed intended to treat?
The device is suitable for cervical and lumbar traction therapy, typically used in orthopedic and rehabilitation settings for spinal decompression and related treatments. Specific diagnoses and treatment plans should be determined by a qualified clinician.
3. How many traction modes and programs does the device offer?
The bed provides eight built-in traction methods (including intermittent, sustained, repeated, and up/down ladder variations) and supports 20 memory treatment plans for convenient clinical operation.
4. What are the main safety features?
Safety features include microcomputer program control of time and force, an emergency stop switch for the patient, and preset limits for traction parameters. Operators should follow the user manual and clinical protocols during use.
5. Who should not use this traction bed (contraindications)?
Contraindications include severe heart, brain, or lung disease; bleeding disorders; severe local skin disease; infectious spinal conditions (e.g., spinal tuberculosis, osteomyelitis), bone tumors, severe osteoporosis, acute spinal injury with cord symptoms; pregnancy beyond three months; intoxication or extreme weakness; and severe uncontrolled hypertension. Mild hypertension patients should limit session duration. Clinician screening is required.
6. What are the cervical traction specifications?
Cervical traction stroke: 0–300 mm; cervical traction force: 0–300 N; angle action range: -10° to +30°. Exact applied parameters should be set per patient needs and clinical guidance.
7. What are the lumbar traction specifications?
Lumbar traction stroke: 0–300 mm; lumbar traction force: 0–990 N. Stroke and force are adjustable within these ranges under program control.
8. What timing and cycle settings are available?
Total traction time can be set from 0 to 99 minutes, traction time 0–9 minutes, intermittent time 0–9 minutes, and training time 1–90 minutes. The microcomputer allows automated program cycles including intermittent and sustained modes.
9. What are the bed dimensions and environmental requirements?
Bed dimensions are 1200 mm (L) × 600 mm (W). Recommended ambient operating temperature range is +5°C to +40°C.
10. What electrical supply does the device require?
Rated voltage: AC 220 V, 50 Hz. Rated input power is 80 VA. Confirm local electrical compatibility before installation.
11. Is special training required to operate the traction bed?
Yes. The device should be operated only by trained clinical personnel who understand traction therapy indications, contraindications, and the unit's controls. Follow local clinical protocols and the manufacturer’s user manual.
12. What maintenance and cleaning are recommended?
Unplug the device before cleaning. Wipe surfaces with a soft, damp cloth and mild disinfectant compatible with the upholstery and avoid wetting motors or electrical components. Perform regular inspections of cables, motors, and fasteners and arrange qualified servicing if faults are detected. Follow the manufacturer’s maintenance schedule in the manual.
13. What is the minimum order quantity (MOQ) and are accessories included?
The listed MOQ is 1 set. Accessory inclusions (e.g., harnesses, straps, power cord) and packaging contents vary by supplier—confirm with the vendor or sales representative for a complete list.
14. Where can I get warranty, service, and technical support?
Warranty terms, after-sales service, and technical support are provided by the manufacturer or authorized dealer. Contact your supplier for warranty details, service agreements, and authorized service centers.
15. How should patient safety be monitored during traction treatment?
Continuously observe the patient for discomfort, neurological changes, or vital sign instability. Ensure the patient knows how to use the emergency stop. Adjust or terminate treatment if adverse symptoms occur, and document the session according to clinical protocols.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading