B21, China Town Mall, Midrand
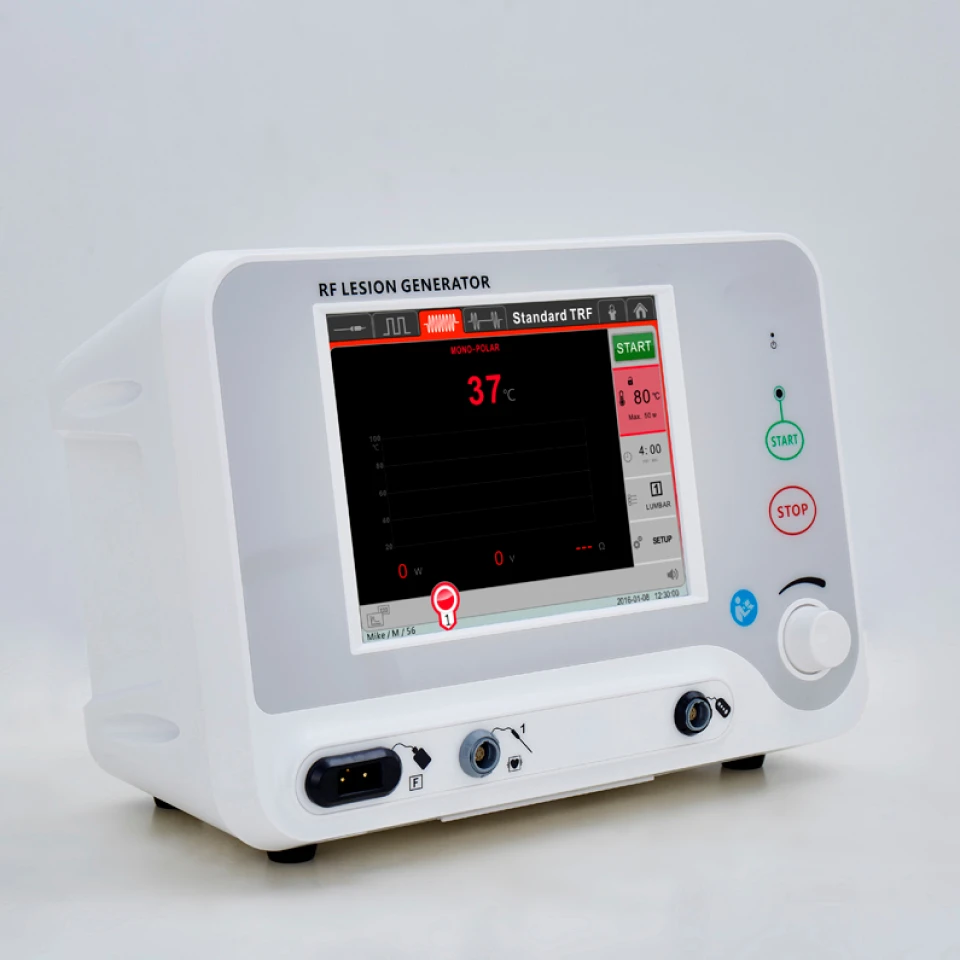
CE Mark Ultrasound Pain Management RF Lesion Generator For Therapy System
- Section : Medical Supplies
- Category : Physical Therapy Equipment
- SKU : 1600991857733

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 20 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the CE Mark Ultrasound Pain Management RF Lesion Generator For Therapy System?
It is a radiofrequency (RF) lesion generator designed for pain management procedures that is intended to be used with ultrasound imaging guidance. The system creates controlled thermal lesions to interrupt pain signaling pathways and is CE marked for sale in the European Economic Area.
2. What clinical indications is this RF lesion generator intended for?
The device is typically used for minimally invasive pain management procedures such as facet joint denervation, peripheral nerve ablation, and other soft-tissue or nerve-targeted RF therapies. Specific indications depend on local regulatory approvals and clinical protocols.
3. What does the CE mark mean for this product?
The CE mark indicates the manufacturer has declared conformity with applicable EU health, safety and environmental protection standards for medical devices and that the product meets the requirements of the EU Medical Device Regulation or applicable directives.
4. How does the RF lesion generator work with ultrasound?
Ultrasound imaging is used to visualize target anatomy and guide needle/electrode placement in real time. Once the electrode is positioned, the RF generator delivers controlled radiofrequency energy to produce a thermal lesion at the target site.
5. What safety features are included in the system?
Common safety features include real-time impedance monitoring, temperature control and limits, automatic shutdown in fault conditions, audible/visual alarms, and user-selectable power/time settings. Operators must follow the device manual and institutional safety protocols.
6. Who should operate this device?
Only qualified healthcare professionals trained in interventional pain procedures, ultrasound-guided interventions, and RF lesioning should operate the system. The manufacturer usually provides training and detailed operating instructions.
7. Are there contraindications or patient factors to consider?
Contraindications typically include infection at the treatment site, bleeding disorders or inability to tolerate anticoagulation interruption, certain implanted electrical devices (e.g., pacemakers) unless assessed by cardiology, and lack of a clear clinical indication. Always review the device instructions for use and consult clinical guidelines.
8. What accessories and consumables are required?
Typical accessories include compatible RF electrodes/needles, grounding/neutral electrodes if required, sterile procedure kits, and ultrasound probes. Some systems may require proprietary electrodes—check the manufacturer’s compatibility list.
9. How do I maintain and clean the system?
Maintenance and cleaning should follow the manufacturer’s instructions for use. Generally that includes regular functional checks, disinfection of external surfaces with approved agents, inspection of cables and connectors, and replacement of consumables. Internal service and calibration should be performed by authorized service personnel.
10. What troubleshooting steps should I try if the generator fails to deliver energy?
Basic checks include verifying power connection and voltage, confirming correct electrode connection and integrity, ensuring grounding or return electrode is properly applied if required, checking for error messages or alarms, and consulting the troubleshooting section of the user manual. Contact technical support if issues persist.
11. Is this device cleared for use outside the EU?
The CE mark reflects conformity with EU requirements. Regulatory status in other regions (such as FDA clearance in the United States) depends on separate approvals. Contact the manufacturer or your local distributor to confirm availability and regulatory clearance in your country.
12. Where can I get training, software updates, or warranty support?
Training, software updates, and warranty details are provided by the manufacturer or authorized distributor. Refer to your purchase documentation for warranty terms and contact your local representative to schedule training or request software/service support.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading