B21, China Town Mall, Midrand
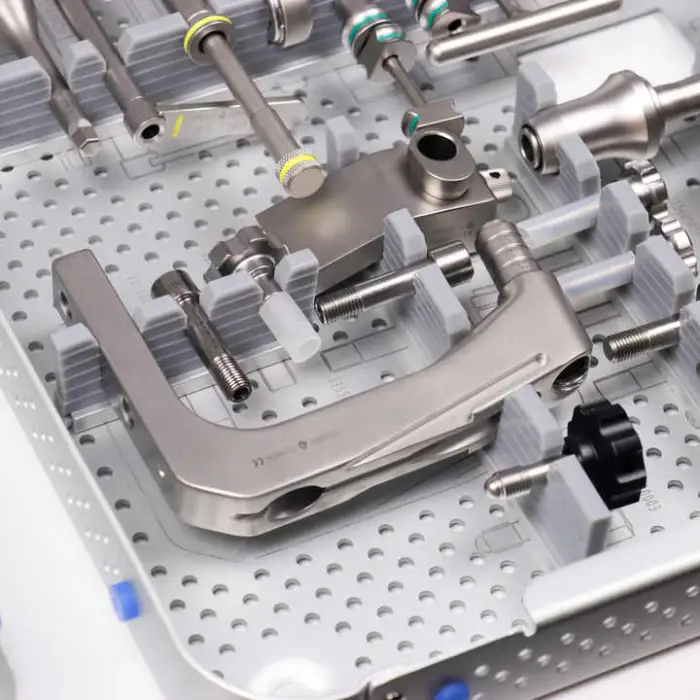
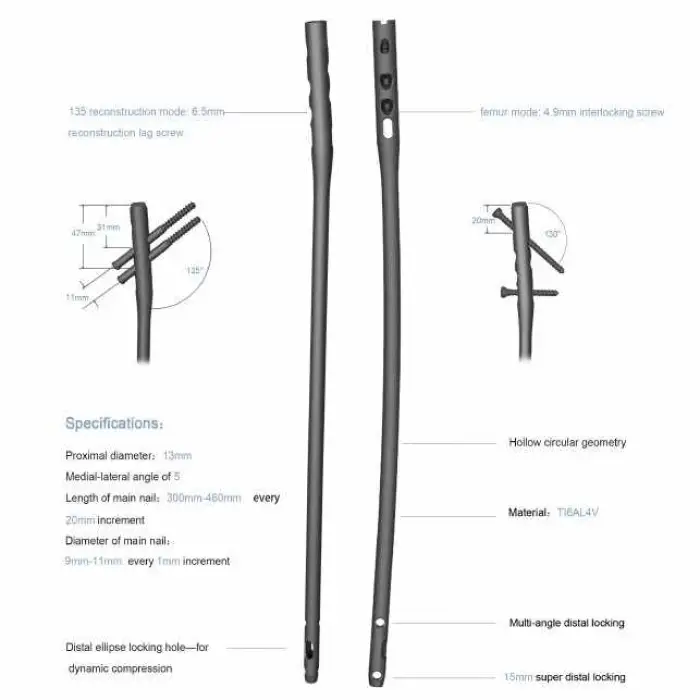
CANWELL Medical Surgical Expert Proximal Femoral Interlocking Nail Orthopedic Instruments
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1600054762702

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 18 Feb, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the primary use of the CANWELL Proximal Femoral Interlocking Nail?
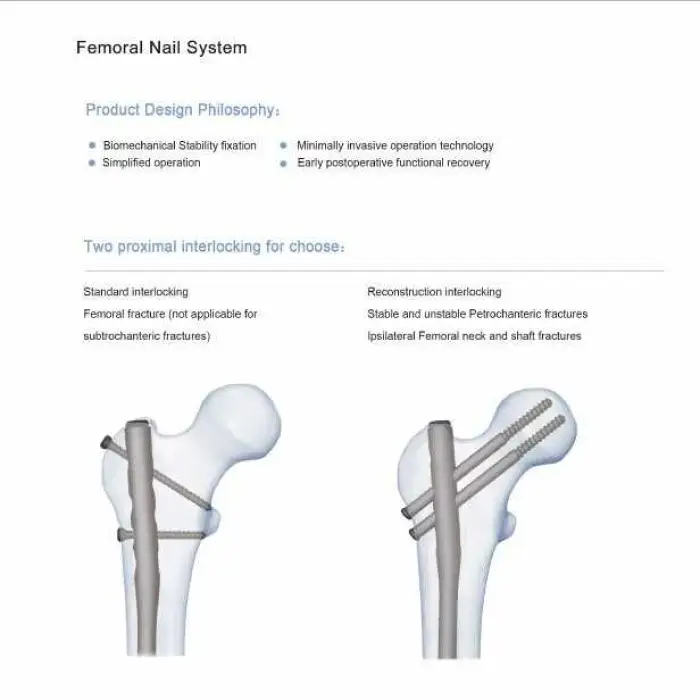
The CANWELL Proximal Femoral Interlocking Nail is primarily used in orthopedic trauma surgery to treat proximal femoral fractures.
2. What materials are used in the construction of this surgical instrument?
The instrument is made from durable stainless steel, ensuring long-lasting use and reliable performance during surgeries.
3. Is the CANWELL Proximal Femoral Interlocking Nail suitable for all patients?
While it is designed for treating proximal femoral fractures, suitability for specific patients should be determined by a qualified orthopedic surgeon.
4. What certifications does this product have?
The CANWELL Proximal Femoral Interlocking Nail is CE and ISO certified, ensuring it meets international quality standards.
5. Does this instrument come with a proximal locking option?
Yes, it includes a proximal locking option with a lag screw, which enhances stability during surgical procedures.
6. What is the classification of this surgical instrument?
The CANWELL Proximal Femoral Interlocking Nail is classified as a Class II instrument.
7. How much does the instrument weigh?
The single gross weight of the CANWELL Proximal Femoral Interlocking Nail is 20.000 kg.
8. What are the dimensions of the packaging for this product?
The single package size is 24x24x14 cm.
9. Can this product be customized with OEM options?
Yes, OEM options are acceptable for the CANWELL Proximal Femoral Interlocking Nail.
10. What kind of surgical techniques does this instrument support?
It supports various surgical techniques aimed at enhancing outcomes in orthopedic procedures for proximal femoral fractures.
11. Is there a specific website where I can find more information about this product?
Yes, you can find more information on the CANWELL website.
12. Is training required to use this instrument effectively?
Yes, proper surgical training is essential to use the CANWELL Proximal Femoral Interlocking Nail effectively and safely.
13. What makes this instrument different from other orthopedic instruments?
Its specific design for proximal femoral fractures, along with its durable stainless steel construction and locking options, sets it apart from other orthopedic instruments.
14. Can this instrument be used in emergency surgeries?
Yes, it is designed for use in orthopedic trauma surgeries, making it suitable for emergency situations involving proximal femoral fractures.
15. What is the recommended storage condition for this surgical instrument?
It is recommended to store the instrument in a dry and sterile environment to maintain its quality and functionality.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading