B21, China Town Mall, Midrand
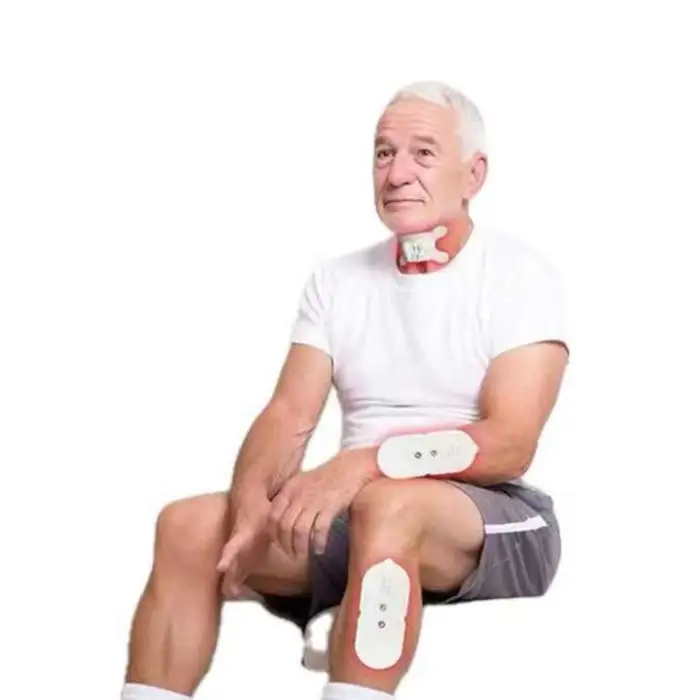
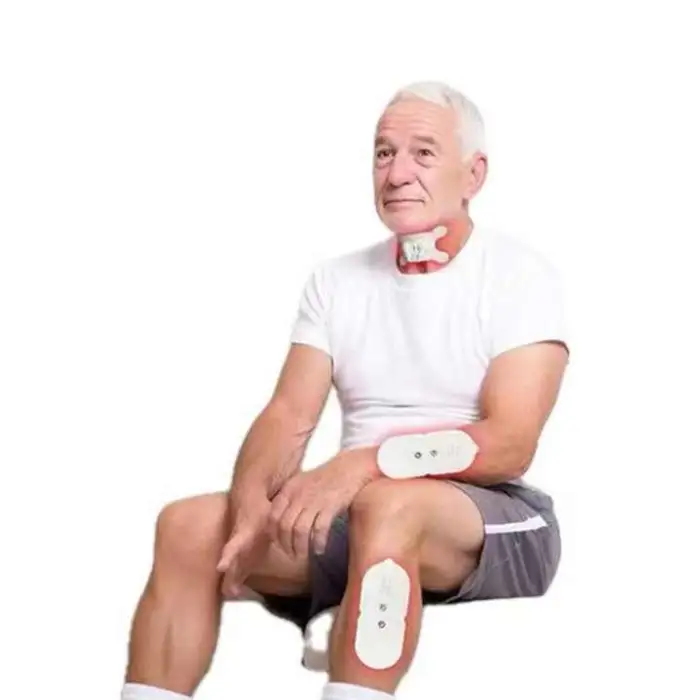
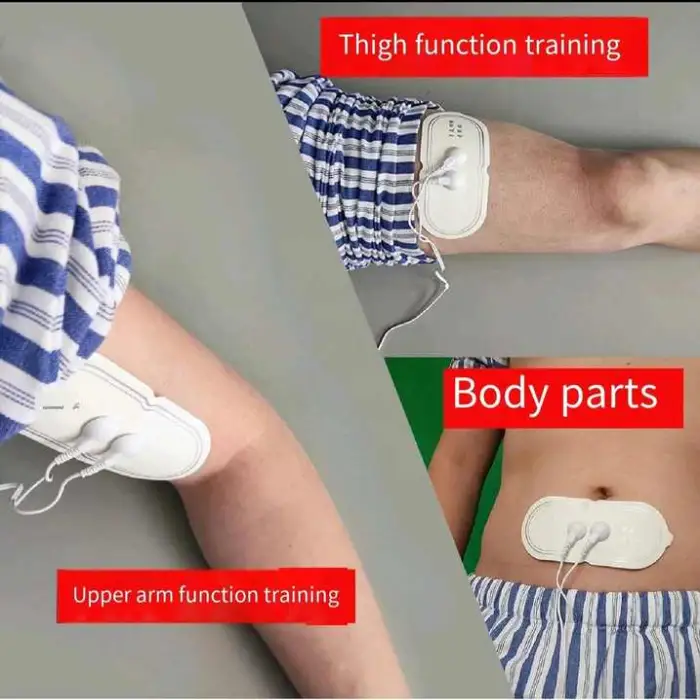
Swallowing Rehabilitation Trainer Elderly Patients Choking Aphasia Stroke Hemiplegia Hands and Legs Physical Therapy Equipment
- Section : Medical Supplies
- Category : Rehabilitation Equipment
- SKU : 1601105435606

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 03 Feb, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
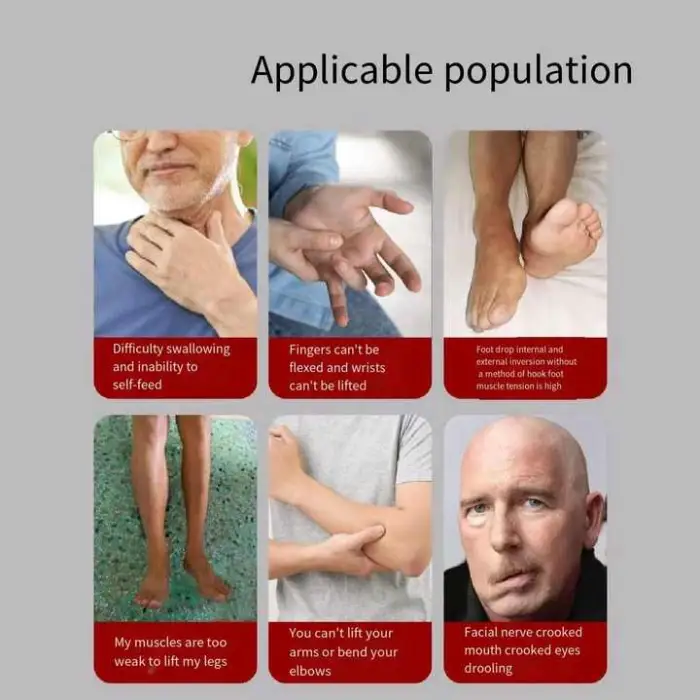
1. What is the Swallowing Rehabilitation Trainer and who is it for?
A portable electrical-stimulation device designed to assist elderly patients with dysphagia (difficulty swallowing). It is intended for use in rehabilitation after choking episodes, stroke, aphasia, facial paralysis or hemiplegia, under clinical guidance.
2. How does the device help improve swallowing?
The trainer uses controlled electrical stimulation to activate muscles involved in swallowing, supporting muscle re-education and coordination. Treatment protocols and intensity should be determined by a therapist or physician.
3. Is this device safe for elderly patients?
When used as directed and under professional supervision it is generally safe. However, there are important contraindications (see next question). Always consult the patient's healthcare provider before use.
4. Are there any contraindications or precautions?
Do not use if the patient has a pacemaker or other implanted electrical device, unstable cardiac conditions, epilepsy, or active infection/open wounds in the treatment area. Use with caution during pregnancy. Always obtain clearance from a physician.
5. Can family members or caregivers operate the trainer at home?
Yes, caregivers can operate it if they receive training and follow a clinician-prescribed protocol. Home use should only be started after professional instruction and ongoing clinical supervision.
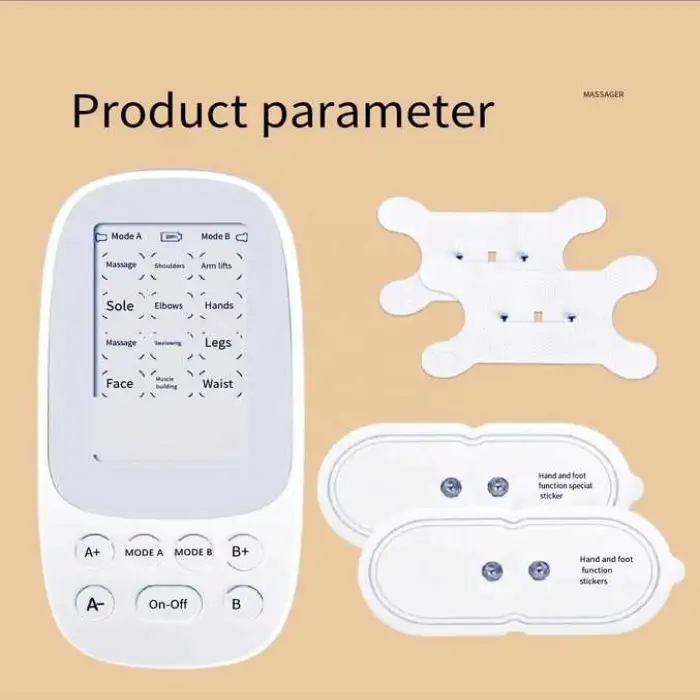
6. What are the device specifications (size, weight, material, color)?
Single package size: 20 x 20 x 20 cm. Single gross weight: 2.000 kg. Material: ABS. Color: white. The unit is designed to be portable.
7. What accessories or consumables are required (electrodes/pads)?
The trainer typically uses adhesive electrode pads to deliver stimulation. Pads are consumables and should be replaced regularly. Check the product listing or package contents to see which accessories are included.
8. How should the device be powered and what is the battery situation?
Power source and battery specifications vary by model. The product description indicates portability but does not list specific battery details—check the seller's product page or user manual for whether it is battery-powered, rechargeable, or uses an AC adapter.
9. How long and how often should therapy sessions be?
Session length and frequency depend on the patient's condition and the therapist's plan. Typical clinical sessions range from 15–30 minutes once or twice daily, but follow the individualized protocol prescribed by a speech-language or rehabilitation therapist.
10. How do I clean and maintain the device?
Turn off and disconnect electrodes before cleaning. Wipe the casing with a soft cloth dampened with mild detergent or disinfectant. Do not immerse the device in water. Replace electrode pads per manufacturer guidance and store unit in a dry, cool place.
11. Is there clinical evidence supporting electrical stimulation for dysphagia?
Electrical stimulation is used in some dysphagia rehabilitation programs and has supporting research for certain patient groups, but outcomes vary. Discuss evidence and expected benefits with a treating clinician and request any available product-specific studies from the manufacturer.
12. Are product samples available?
Yes — the product listing indicates samples are available. Contact the seller or distributor to request a sample and to learn about any terms or qualification requirements.
13. What certifications, approvals or quality standards does the device have?
Certification information (CE, FDA, ISO, etc.) is not provided in the basic description. Ask the seller for documentation of regulatory approvals and quality certificates relevant to your country before purchase.
14. Does the device come with a warranty and technical support?
Warranty and after-sales support details are not specified here. Check the product listing or contact the vendor directly to confirm warranty period, return policy and available technical/clinical support.
15. Where can I buy the Swallowing Rehabilitation Trainer and how do I arrange clinician training?
Purchase through the manufacturer, authorized distributors or the seller's sales channels. For training, request clinical training resources or referrals to a speech-language pathologist or rehabilitation therapist who can provide hands-on instruction and a treatment plan.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals