B21, China Town Mall, Midrand
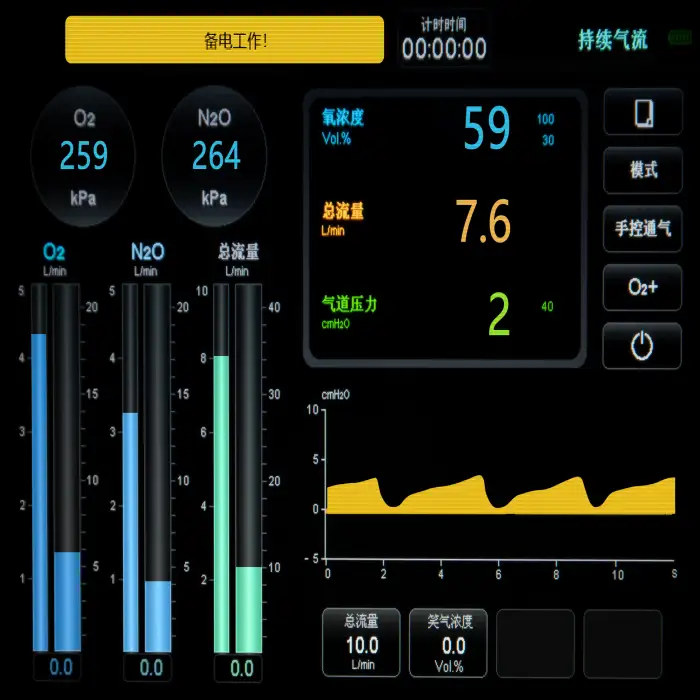
Superstar S8800C Nitrous Oxide Sedation System for Surgery
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1600815319819

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 21 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Superstar S8800C Nitrous Oxide Sedation System?
The Superstar S8800C is an electrical nitrous oxide (N2O) sedation system designed to provide controlled N2O delivery for pain management during surgical and emergency care procedures in hospitals and clinics.
2. What clinical applications is the S8800C intended for?
It is intended for use in surgical and emergency care settings to provide fast-acting, controlled nitrous oxide sedation for short-term pain and anxiety management. Use should be limited to procedures and patients deemed appropriate by qualified clinicians.
3. What certifications and standards does the product meet?
The Superstar S8800C is CE and ISO certified and meets applicable safety requirements referenced, including GB15979-2002, as stated by the manufacturer.
4. What are the primary safety features of the system?
Key safety elements include controlled N2O delivery mechanisms for precise sedation dosing, design and construction compliant with stated safety standards, and emergency care functionality intended for rapid response in critical situations. Operators must follow the user manual and institutional protocols.
5. What power source does the S8800C require?
The system is electrically powered for consistent performance. Specific voltage, frequency, and plug type information are provided in the product manual or technical datasheet—confirm these details with the manufacturer or supplier before installation.
6. What materials is the system made from and how durable is it?
The unit is constructed from a combination of acrylic, metal, plastic, steel, wood, and resin. These materials are selected for durability and long-term performance in clinical environments; follow manufacturer care instructions to preserve longevity.
7. What is the shelf life of the S8800C?
The stated shelf life for the Superstar S8800C is 3 years. For devices kept in storage, follow manufacturer recommendations regarding storage conditions and perform pre-use checks after storage.
8. How should the S8800C be cleaned and disinfected?
Cleaning and disinfection must follow the manufacturer’s instructions. Because the unit contains mixed materials (including acrylic and wood), avoid harsh solvents or immersion unless expressly permitted. Use approved disinfectants and techniques outlined in the user manual.
9. What maintenance and calibration are required?
Regular maintenance, inspection, leak testing, and periodic calibration are required to ensure safe operation. Specific schedules and procedures are provided in the service manual; maintenance should be performed by trained technicians or authorized service agents.
10. Are there recommended training or credentialing requirements for operators?
Yes. Only qualified healthcare professionals trained in nitrous oxide sedation and emergency airway management should operate the system. Follow local regulations and institutional credentialing requirements.
11. Are there contraindications or precautions for using nitrous oxide with this system?
Contraindications and precautions are the same as for nitrous oxide therapy generally (for example, certain respiratory conditions, bowel obstruction, recent eye surgery using gas, or other clinician-determined risks). Refer to clinical guidelines and the device’s instructions for use; clinical judgment and patient assessment are required.
12. What gas supplies and consumables does the S8800C require?
The system requires medical-grade nitrous oxide and may require oxygen supply or other consumables depending on configuration. Consult the product technical specifications or supplier for compatible gas cylinders, fittings, tubing, masks, and disposables.
13. How is the S8800C installed and set up?
Installation should be performed per the manufacturer’s installation guide by qualified personnel. Typical requirements include a suitable electrical outlet, secure placement, connection to approved medical gas sources (if applicable), and pre-use safety checks.
14. What accessories or optional components are available?
Accessory offerings (masks, hoses, mounting stands, monitoring add-ons, consumables, service kits) vary by supplier. Contact the manufacturer or authorized dealer for an up-to-date list of compatible accessories and part numbers.
15. What warranty and after-sales support are available?
Warranty terms and after-sales support vary by distributor and region. Consult your sales contract, product documentation, or contact the manufacturer/authorized reseller for warranty duration, coverage details, and technical support or service center information.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading