B21, China Town Mall, Midrand
Portable Physiotherapy Physical Therapy Equipment Pain Relief Ultrasound Therapy Machine
- Section : Medical Supplies
- Category : Rehabilitation Equipment
- SKU : 1600938639087

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 21 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
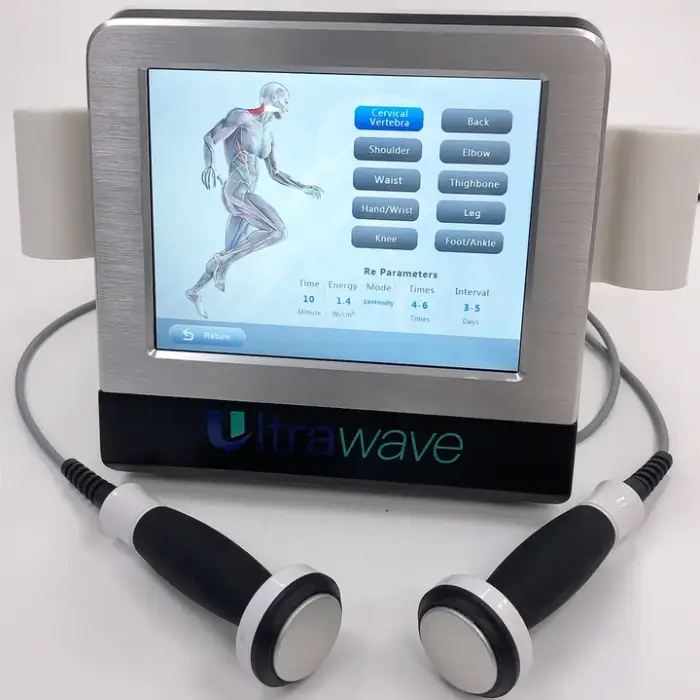
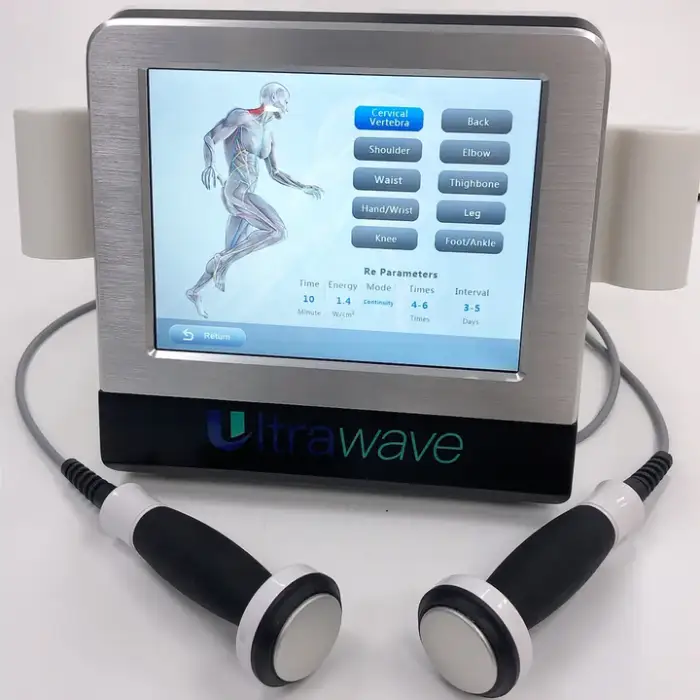
1. What is the Portable Physiotherapy Ultrasound Therapy Machine (KF-WAVE)?
The KF-WAVE is a desktop, portable ultrasound therapy device designed to provide pain relief and support rehabilitation. It delivers therapeutic ultrasound to promote healing and soothe sore muscles and joints for home users, physiotherapy clinics, and sports rehab settings.
2. Which body areas can I treat with this device?
The device is suitable for multiple target areas including the body, legs/arms, neck/throat, feet, and hands. Treatment should follow the user manual or a clinician's guidance for each specific area.
3. Who can use this machine?
It is intended for use by adults at home and by trained professionals in clinics and sports rehabilitation. Always consult a healthcare professional before starting treatment, especially if you have underlying health conditions.
4. How does ultrasound therapy work?
Therapeutic ultrasound uses high-frequency sound waves to deliver mechanical energy into tissues, which can increase local blood flow, reduce pain, and support soft tissue healing. Results vary by condition and individual.
5. What are the key features of this product?
Key features include a compact, portable desktop design, free spare parts included, online and video technical support for setup, and applicability to multiple body areas for pain relief and rehabilitation.
6. What is included in the package?
Typical shipments include the main device (model KF-WAVE), free spare parts, a power cord with the region-appropriate plug, a user manual, and packaging (carton or flight case). Exact contents may vary—confirm with the seller before purchase.
7. Which plug types and power compatibility are available?
Plug types available include AU, UK, EU, US, and CN. Voltage and frequency support may vary by model — check the product label or ask the seller for region-specific electrical specifications before use.
8. How heavy and portable is the machine?
The unit weighs approximately 8.0 kg and is designed as a compact, portable desktop device. It can be transported in its carton or flight case for clinical or travel use.
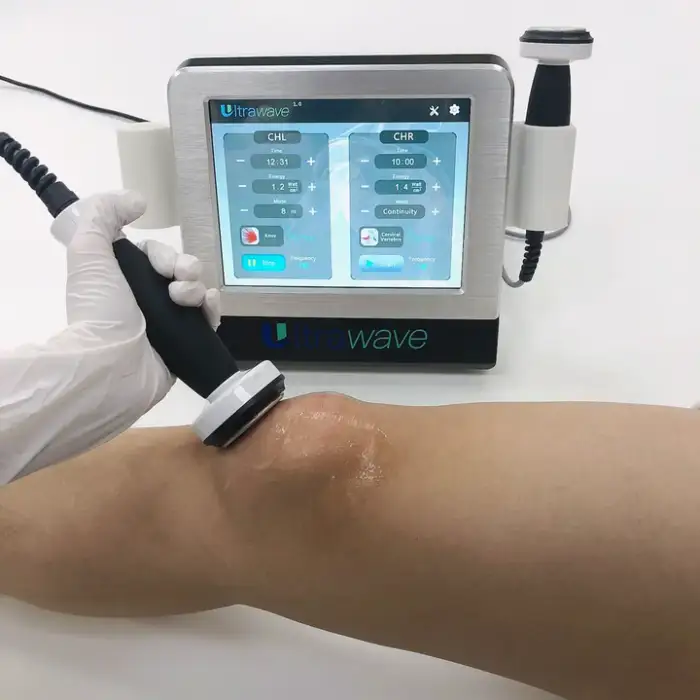
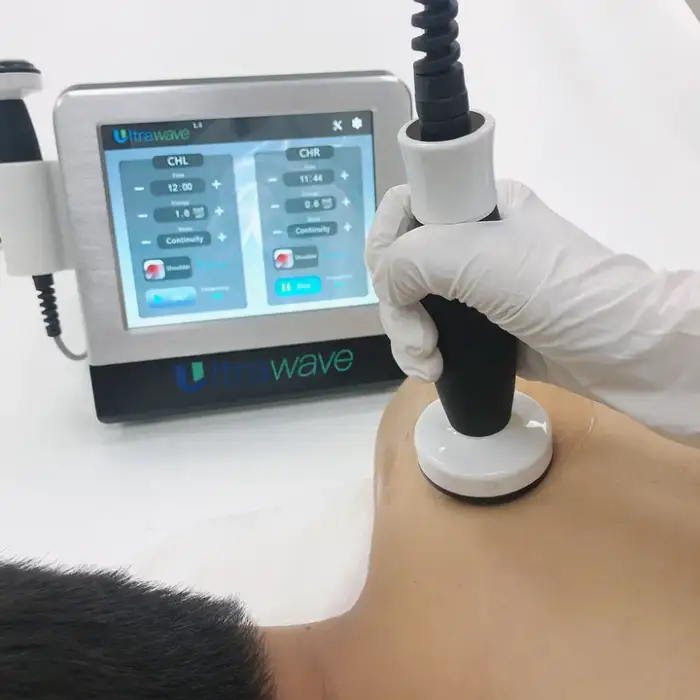
9. How do I set up and start a treatment?
Basic setup involves connecting the appropriate power cord, attaching the therapy transducer per the manual, applying coupling gel to the treatment area, selecting a program or intensity, and following session time guidelines. Video technical support is available for step-by-step setup.
10. What treatment settings and session durations should I use?
Settings and duration depend on the clinical indication, treatment area, and patient tolerance. Follow the manufacturer's instructions and your clinician's recommendations. If unsure, consult a healthcare professional before adjusting settings.
11. Are there any safety warnings or contraindications?
Common contraindications include use over pregnancy (abdomen/uterus), malignant tumors, active infections, open wounds, over eyes or reproductive organs, and directly over implanted electronic devices (e.g., pacemakers) or growth plates in children. Always read safety warnings in the manual and consult a clinician if you have medical concerns.
12. How should I clean and maintain the device?
Clean the transducer and device surfaces with a soft cloth and manufacturer-approved disinfectant. Do not immerse the device or transducer in liquid. Regularly inspect cables and connectors and store the unit in its carrying case when not in use. Refer to the maintenance section of the manual for specifics.
13. What technical support and spare parts are provided?
The product comes with free spare parts and offers online and video technical support for setup and troubleshooting. For repairs beyond included parts, contact the seller or authorized service center.
14. Is there a warranty and what does it cover?
Warranty terms vary by seller and region. Some purchases include spare parts and technical support, but warranty length and coverage (parts, labor, shipping) should be confirmed with the seller or distributor at the time of purchase.
15. Does the device have medical certifications (CE/FDA) or approvals?
Certification status is not listed here. If you require CE, FDA, or other regulatory approvals, request the device's certification documents from the seller or manufacturer before purchasing or using it in a regulated clinical setting.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading