B21, China Town Mall, Midrand
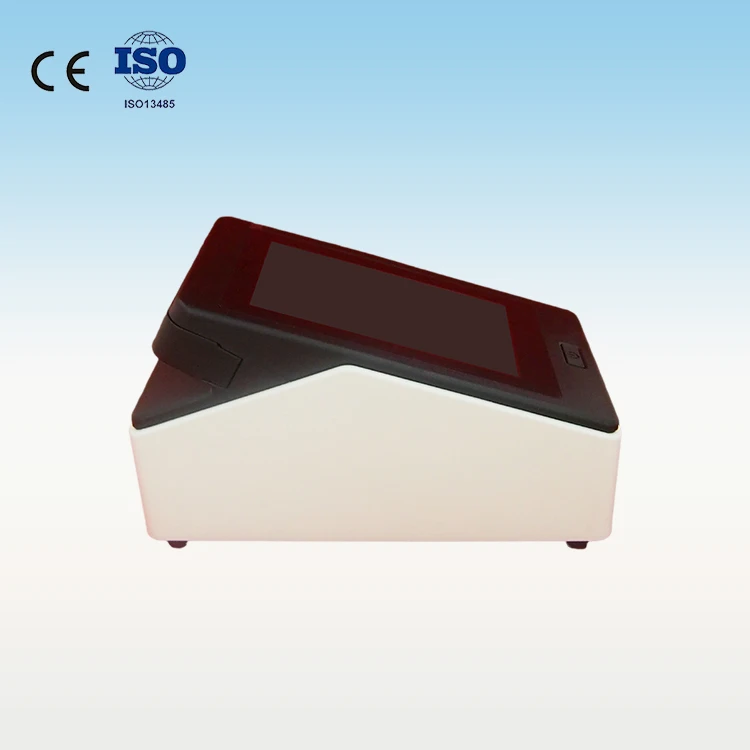
Portable Fluorescence Immunoassay Analyzer for Clinics
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1600698894164

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 21 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the WIZ-A101 Portable Fluorescence Immunoassay Analyzer?
The WIZ-A101 is a compact, portable diagnostic instrument designed for clinical use that performs rapid fluorescence immunoassay testing using single-use cassettes to deliver reliable results at the point of care.
2. What testing technology does this analyzer use?
It uses advanced fluorescence immunoassay (FIA) technology to detect target analytes with high sensitivity and specificity.
3. What sample types can be tested?
Compatible sample types depend on the specific assay cassette; commonly supported matrices include whole blood (fingerstick), serum, plasma, urine or saliva. Refer to the cassette/test insert for approved sample types.
4. How fast are the test results?
The analyzer delivers results within minutes. Exact turnaround time varies by assay — typical runtimes range from about 5 to 30 minutes depending on the test.
5. How many tests can the device run at once or per hour?
The WIZ-A101 processes one cassette at a time. Hourly throughput depends on assay runtime (for example, a 10-minute assay could enable up to ~6 tests per hour performed sequentially).
6. What consumables are required?
The system uses single-use disposable test cassettes specific to each assay. Additional consumables may include pipettes, sample transfer devices, and control materials. Use only manufacturer-approved consumables for validated performance.
7. What are the power and portability options?
The unit is designed to be compact and lightweight for clinic and mobile use. Power options and battery availability depend on the configured model — consult the product specifications or contact sales for details about AC vs. battery operation and run time.
8. How does the device handle data and connectivity?
Data handling and connectivity options (such as USB, Ethernet, Wi‑Fi or serial output) depend on model and configuration. Contact the manufacturer for available interfaces and data export formats.
9. What quality and regulatory certifications does the WIZ-A101 have?
The product is ISO certified, indicating compliance with international quality management standards. Local regulatory approvals (CE, FDA clearance/510(k), etc.) vary by market and assay — check with the manufacturer for specific regulatory status in your region.
10. What warranty and after-sales support are provided?
The WIZ-A101 is supplied with a 24-month warranty. OEM and ODM customization and customizable service plans are available; technical support and maintenance packages can be arranged through the manufacturer or authorized distributor.
11. Is training required to operate the analyzer?
The system is designed for ease of use with streamlined cassette workflows, but operator training is recommended. The manufacturer typically provides user manuals and can provide on-site or remote training and technical support.
12. How is calibration and quality control performed?
Routine QC and calibration procedures depend on the assays in use. Users should run manufacturer-recommended control materials at the frequency specified in the assay inserts and follow the operator manual for calibration and verification steps.
13. What are the storage and shelf-life requirements for cassettes?
Storage conditions and shelf life vary by assay and are printed on the cassette packaging and package insert. Follow the labeled storage temperature and expiration date to ensure reliable results.
14. What maintenance and cleaning are required?
Regular basic cleaning and inspection should be performed per the user manual. Preventive maintenance intervals and any required service by authorized technicians are described in the service documentation. Use only recommended cleaning agents and procedures.
15. How do I order or request OEM/ODM customization?
Contact the manufacturer's sales team or an authorized distributor to discuss ordering, pricing, lead times and available OEM/ODM customization options. Provide your clinical requirements so they can propose appropriate assay and hardware configurations.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading