B21, China Town Mall, Midrand
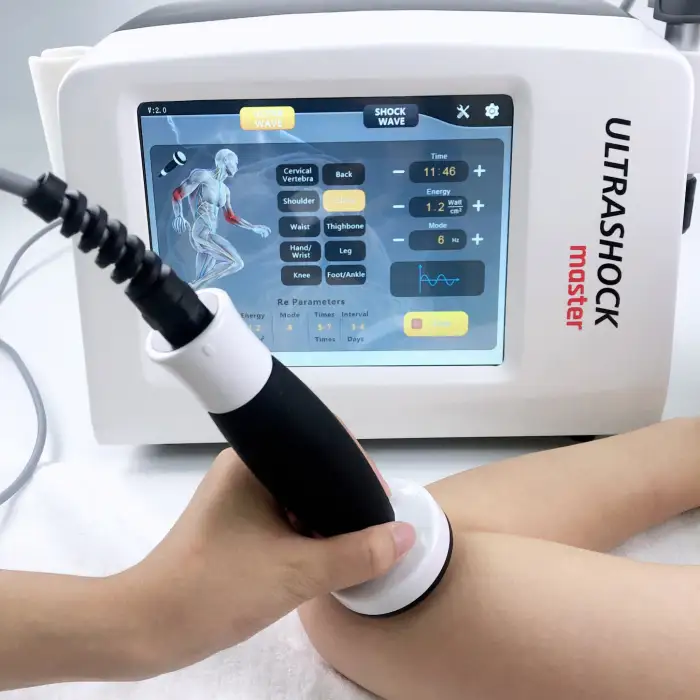
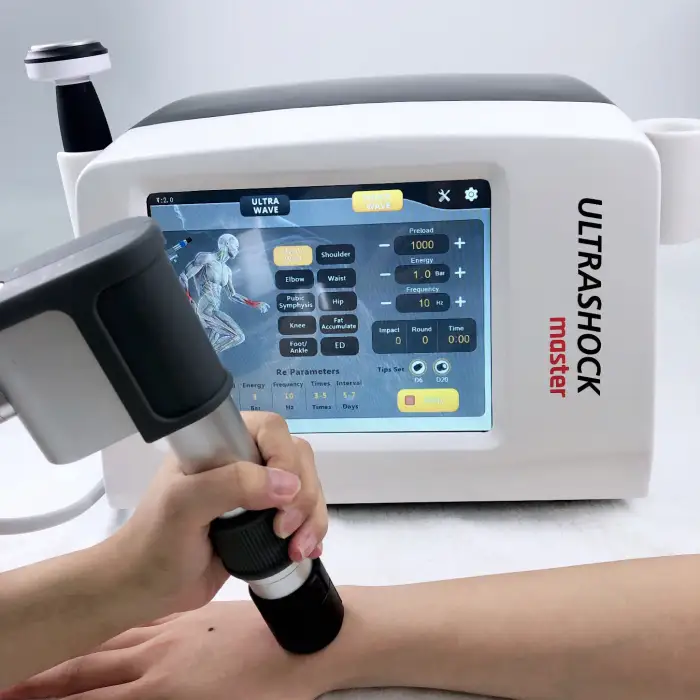
Pneumatic Shockwave + Ultrasound Machine – JN-USM Model
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1601014827908

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 21 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Pneumatic Shockwave + Ultrasound Machine – JN-USM Model?
The JN-USM is a combined physiotherapy device that integrates pneumatic shockwave therapy and therapeutic ultrasound into a single unit to support pain relief, soft-tissue rehabilitation, musculoskeletal recovery and accelerated healing in clinic or supervised home settings.
2. How do the shockwave and ultrasound therapies differ and why are they combined?
Shockwave delivers focused mechanical pressure pulses to break adhesions, reduce calcifications and stimulate tissue repair, while ultrasound converts acoustic energy into deep heat to increase circulation and metabolism. Combining both modalities allows complementary effects—mechanical stimulation plus thermal/biological enhancement—for more comprehensive treatment.
3. What clinical conditions can the JN-USM be used to treat?
Common indications include delayed fracture healing and nonunion, early femoral head necrosis, calcific supraspinatus tendonitis, frozen shoulder, long head of biceps tendinitis, lateral/medial epicondylitis (tennis/golfer's elbow), plantar fasciitis, insertional Achilles tendinopathy and some lumbar disc-related symptoms when indicated by a clinician.
4. What are the key technical specifications I should know?
Shockwave energy: 1–6 BAR; shockwave frequency: 1–21 Hz; shockwave working head with 12 interchangeable tips; tip life: ~5 million pulses. Ultrasound energy: 1–3 W/cm² at 1 MHz. Machine power: 250 W. Interface: 10.4-inch touchscreen.
5. How should I choose shockwave and ultrasound treatment settings?
Start with lower energy and frequency settings and increase gradually based on patient tolerance and clinical response. Use lower shockwave energies for sensitive areas and higher energies for chronic calcifications; ultrasound intensity and duration depend on tissue depth and goals. Follow established treatment protocols or a clinician's guidance.
6. What is a typical treatment duration and recommended session frequency?
Typical sessions last 5–20 minutes depending on modality and condition. Shockwave courses often include 3–6 sessions spaced 3–7 days apart; ultrasound can be applied more frequently (e.g., 2–3 times weekly) depending on the protocol. Individual plans should be set by a treating clinician.
7. What are the main contraindications and precautions?
Contraindications commonly include pregnancy (over abdomen/pelvis), active infection or malignancy at the treatment site, over untreated growth plates in children, presence of an untreated blood clot or thrombus, and areas with impaired sensation or implanted electronic devices (consult manufacturer/physician if pacemaker present). Use caution if the patient is on anticoagulants and always screen patients prior to treatment.
8. Is this device approved for home use?
The JN-USM is classed as a Class I medical device, which meets basic safety standards; however, safe and effective use requires training. Home use should be under the direction of a healthcare professional or after adequate device/operator training and follow-up.
9. What accessories and consumables are included or required?
The system uses interchangeable shockwave tips (12 supplied tip types) and ultrasound coupling gel. Other consumables include replacement tips over long-term use and routine cleaning supplies. Confirm the exact included accessories with the supplier.
10. How durable are the shockwave tips and what is 'bullet life'?
'Bullet life' refers to the expected durability of the shockwave applicator tips—specified as approximately 5 million pulses for the JN-USM—after which tips should be inspected and replaced if wear affects performance.
11. How do I clean and maintain the device?
Turn off and unplug before cleaning. Wipe external surfaces and the touchscreen with a soft cloth dampened with mild detergent or manufacturer-approved disinfectant. Clean the ultrasound transducer with recommended gel-removal procedures and disinfect shockwave applicators per manufacturer instructions. Schedule periodic professional servicing as recommended.
12. What power and installation requirements does the machine have?
The device has a 250 W power rating. Confirm local mains voltage compatibility and ensure a stable power supply and properly grounded outlet. For clinic installations, allow adequate space for ventilation and operator access to the 10.4-inch touch screen.
13. Are there side effects or risks I should warn patients about?
Common transient effects include mild-to-moderate discomfort during treatment, temporary redness, bruising, soreness or local swelling. Serious complications are rare when used correctly, but any unexpected or severe reactions should prompt cessation and medical review.
14. Do operators require special training or certification to use the JN-USM?
Yes. Operators should receive training on both shockwave and therapeutic ultrasound principles, device settings, safety protocols and patient selection. Many jurisdictions require a licensed healthcare professional to operate such devices; check local regulations and supplier training programs.
15. What warranty, technical support or spare parts options are available?
Warranty terms, technical support and spare part availability vary by supplier and region. Contact the vendor or manufacturer for details on warranty period, service contracts, replacement tips and authorized service centers.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading