B21, China Town Mall, Midrand
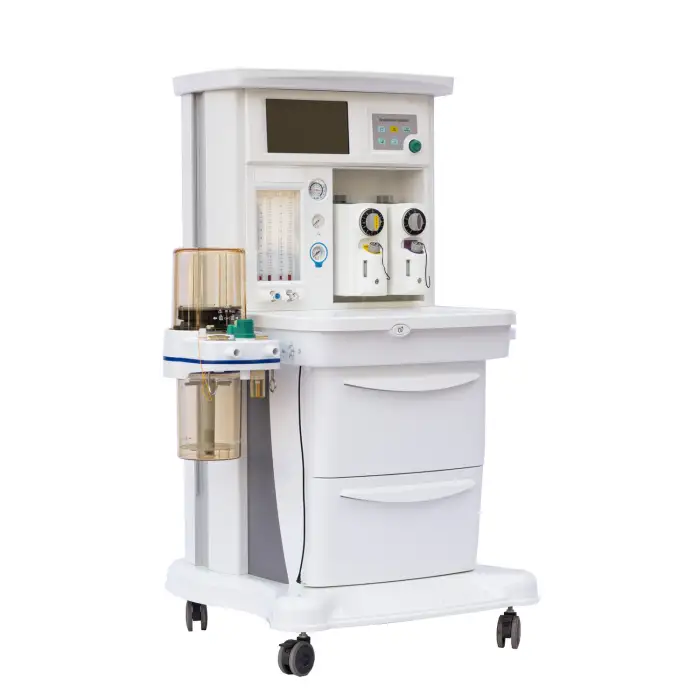
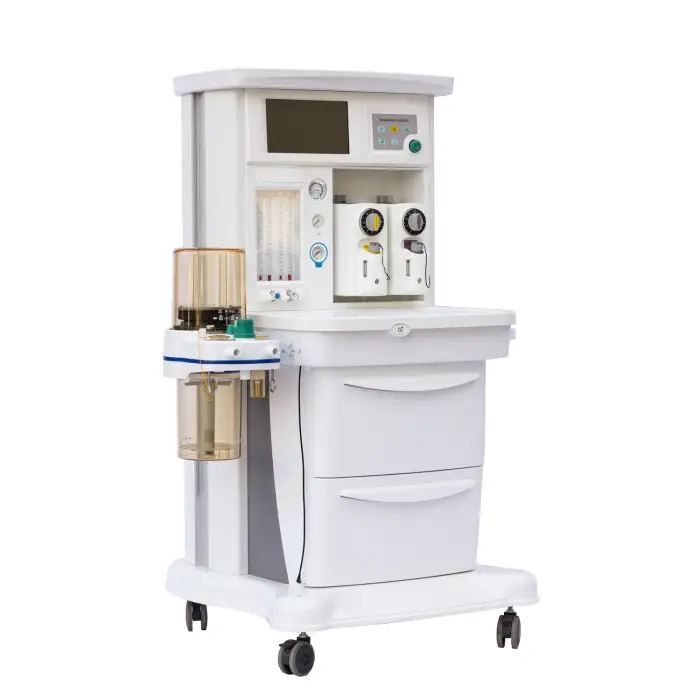
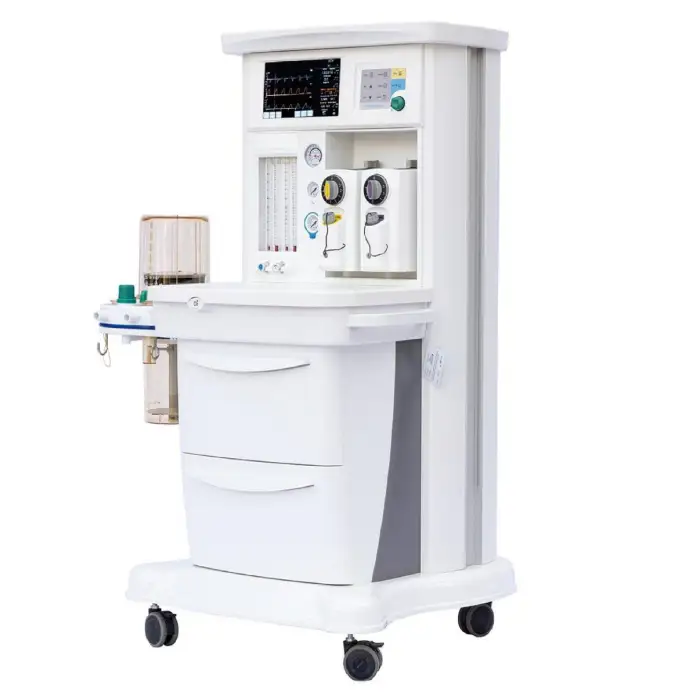
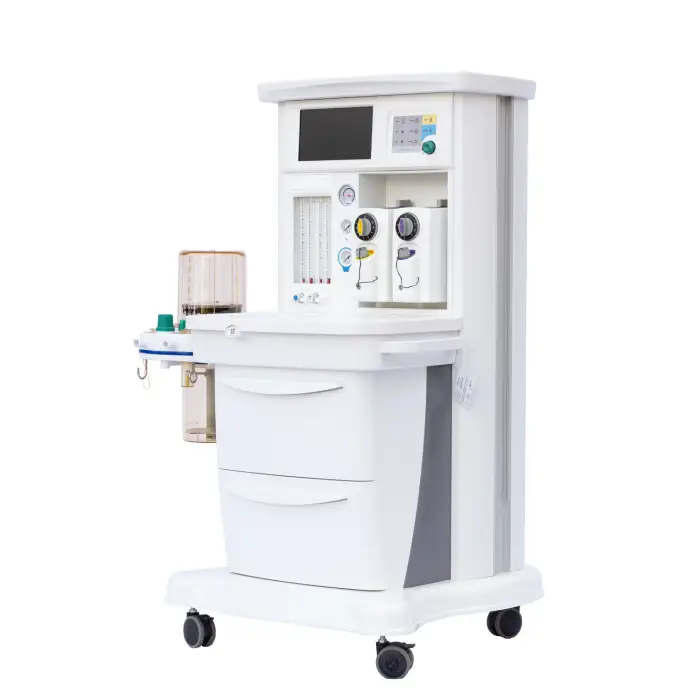
MSLGA25 Trolley Electronically Controlled Anesthesia Machine
- Section : Medical Supplies
- Category : Medical X-ray Equipments
- SKU : 1601259092215

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 02 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the MSLGA25 Trolley Electronically Controlled Anesthesia Machine?
The MSLGA25 is an electronically controlled anesthesia workstation on a trolley platform designed to deliver safe and precise general anesthesia. It features a 10.1-inch touch screen, compact design, two vaporizers, multiple ventilation modes, and additional functions like pressure/flow triggering, altitude compensation and an integrated headlight.
2. What size and type of display does the MSLGA25 have?
The machine includes a 10.1-inch capacitive touch screen designed for intuitive monitoring and control of ventilation and anesthesia parameters.
3. Which gas sources are supported by the MSLGA25?
The MSLGA25 supports Air, Oxygen (O2) and Nitrous Oxide (N2O) as gas sources.
4. How many vaporizers does the unit have and what are they used for?
The unit is equipped with two vaporizers, allowing use of two different volatile anesthetic agents for flexible clinical workflows. For specific agent compatibility and mounting instructions, consult the product manual or supplier.
5. Which ventilation modes are available on the MSLGA25?
Available ventilation modes include VCV (Volume-Controlled Ventilation), A/C (Assist/Control), SIMV (Synchronized Intermittent Mandatory Ventilation), MANUAL, PCV (Pressure-Controlled Ventilation), PSV (Pressure Support Ventilation) and STAND-BY.
6. What tidal volume range does the MSLGA25 support?
Tidal volume is adjustable from 20 mL up to 1500 mL, covering neonatal, pediatric and adult ventilation needs.
7. What triggering options does the machine provide?
The MSLGA25 includes pressure and flow trigger functions to detect spontaneous patient efforts and assist synchronization between the patient and the ventilator.
8. What is altitude compensation and why is it important?
Altitude compensation adjusts gas delivery and sensor calibrations to maintain accurate ventilation and anesthetic delivery at different elevations. This helps ensure consistent performance and safety when operating the machine at higher or lower altitudes.
9. Does the MSLGA25 include lighting to assist procedures?
Yes, the unit includes an integrated headlight to improve visibility during airway management and other procedures.
10. Is the MSLGA25 suitable for use in hospitals, outpatient clinics and emergency services?
Yes. The machine’s compact trolley design and versatile modes make it suitable for general hospital operating rooms, outpatient surgical centers and emergency medical settings. Always verify site-specific requirements and local regulations before deployment.
11. Is the MSLGA25 battery-powered or portable for transport?
The product description lists it as a trolley-mounted anesthesia machine. Battery or internal backup power is not specified in the provided information—check the technical manual or contact the supplier for details about portability and uninterrupted power options.
12. What alarms and safety monitoring features does the MSLGA25 provide?
The description specifies pressure and flow triggering and altitude compensation, but it does not list the complete alarm suite. For a full list of alarms (e.g., disconnection, high/low pressure, apnea, gas supply alerts) and configurable limits, consult the product manual or contact the manufacturer.
13. How should the MSLGA25 be cleaned and maintained?
Follow your facility’s infection control policies and the manufacturer’s maintenance and cleaning instructions. The user manual will provide recommended cleaning agents, disassembly/reassembly guidance for components (breathing circuits, vaporizers, filters), preventive maintenance schedules and calibration procedures.
14. Is the MSLGA25 compatible with standard breathing circuits and filters?
The machine is intended for standard clinical use and typically will accept commonly used breathing circuits and disposable filters. For confirmation of specific connector types and compatible accessories, review the technical specifications in the manual or contact your supplier.
15. Where can I get technical support, warranty information or regulatory certifications for the MSLGA25?
For warranty terms, technical support, service, spare parts and certification details (CE, FDA clearance, ISO, or local approvals), contact the manufacturer or the authorized distributor. They can provide documentation specific to your region and unit configuration.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading