B21, China Town Mall, Midrand
mobile hospital baby care equipment electric Infant Incubator
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1600211182274

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 21 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
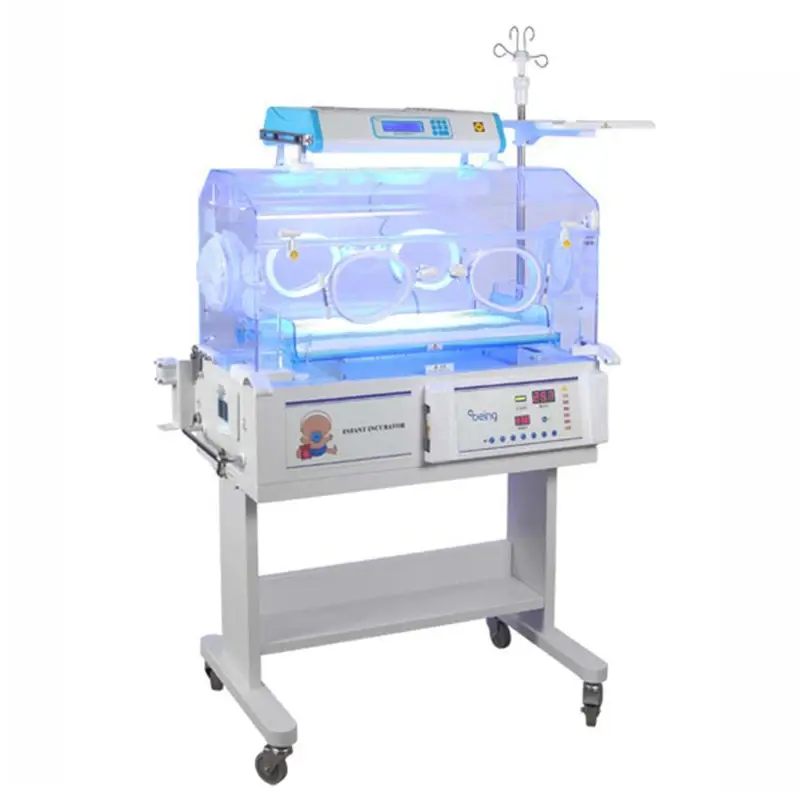
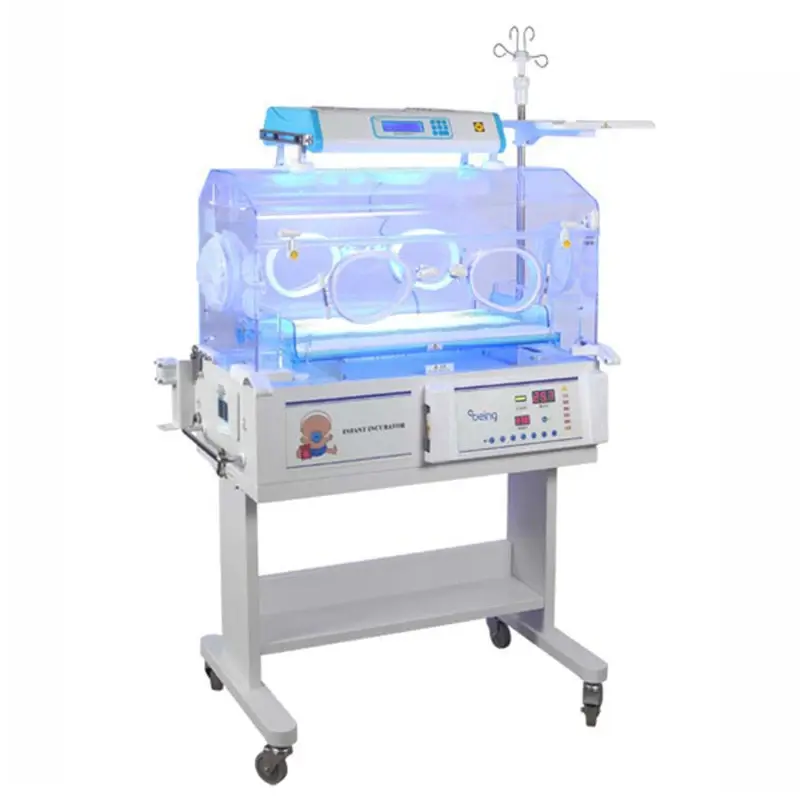
1. What is the TOPNITH TN-3000A Mobile Electric Infant Incubator?
The TN-3000A is a mobile hospital-grade electric infant incubator designed to provide a controlled thermal and humid environment for newborns. It uses advanced, computer servo-controlled air-temperature regulation and offers options such as phototherapy, mattress tilting, and data connectivity.
2. How does the incubator control temperature?
Temperature is controlled by a computer-driven air temperature servo system that precisely regulates the incubator air and mattress environment to maintain setpoints reliably.
3. What are the temperature ranges and alarm thresholds?
Temperature modes: Air Mode 25.0℃ to 35.0℃; High Temperature Mode 37.1℃ to 39.0℃. Air over-temperature threshold is below 38.0℃ and the high-temperature mode alarm is below 40.0℃. The system includes temperature alarms to alert staff if limits are exceeded.
4. What is the temperature accuracy and mattress uniformity?
Air control accuracy is within ±0.5℃. Mattress temperature uniformity is ≤0.8℃.
5. Does the incubator provide humidity control?
Yes — the unit includes a removable humidity reservoir to support humidity control. The reservoir is designed for easy filling and cleaning; specific humidity range settings should be confirmed with the manufacturer or product manual.
6. What alarms and safety features are included?
The TN-3000A includes self-checking diagnostics and temperature alarms (including over-temperature alarms). These alert clinical staff to system faults or environment deviations for prompt action.
7. Is phototherapy supported?
Phototherapy is available as an optional feature to support jaundice treatment. Verify the specific phototherapy module and wavelengths with your supplier.
8. Does the incubator support mattress tilting/positioning?
Mattress tilting adjustment is offered as an optional free-step feature, allowing flexible positioning when specified.
9. What connectivity options are available for data management?
An RS-232 connector is provided for connectivity to external data management systems and monitoring equipment. Integration details depend on your facility's IT and monitoring setup.
10. What are the power requirements and power consumption?
The incubator operates on mains electricity: 220V 50Hz to 230V 60Hz. Power consumption is ≤450 VA.
11. How quiet is the incubator in operation?
The TN-3000A operates with a noise level of ≤55 dB(A), providing a calm environment for newborns.
12. What materials and safety certifications does the product have?
The unit is constructed from metal, plastic and steel. It is classified as Class II medical equipment, complies with GB2626-2006 safety standards, and has TUV quality certification.
13. Is the TN-3000A truly mobile and suitable for intra-hospital transport?
The product is described as mobile for use within hospital settings. For specific transport use (e.g., moving between departments or to imaging suites) confirm mobility features and any institutional transport protocols with your supplier and clinical engineering team.
14. How should the incubator be cleaned and maintained?
The removable humidity reservoir facilitates cleaning. Follow the manufacturer's cleaning and disinfection procedures in the user manual. Routine maintenance, functional checks, and periodic calibration by qualified technicians are recommended; consult the supplier for a maintenance schedule.
15. What is the warranty or shelf life and how do I get service?
The product lists a shelf life of 1 year and carries TUV certification. Warranty terms and service contracts vary by distributor and region; contact your TOPNITH supplier or authorized service center for warranty details, service plans, and technical support.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading