B21, China Town Mall, Midrand
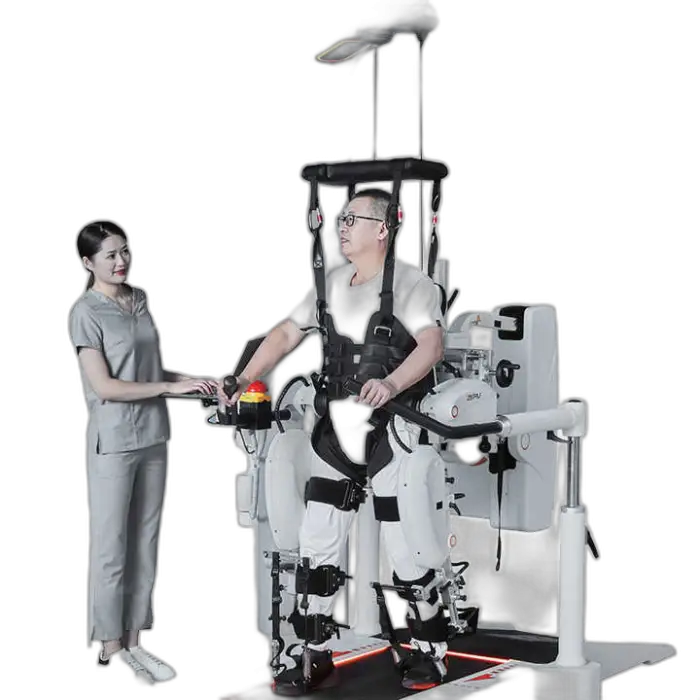
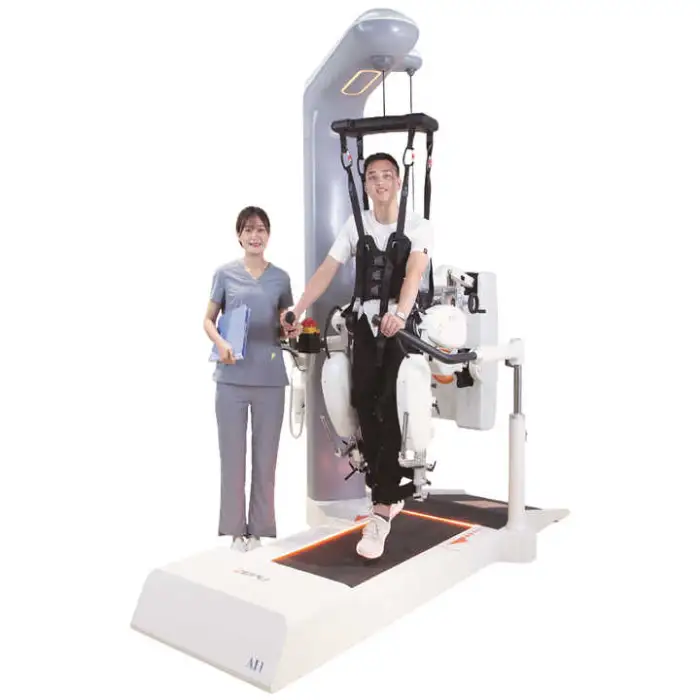
Medical Rehabilitation Equipment Gait Training Robotic for Stroke Patients Walking Exercise Neuro Rehabilitation Robot Hospital
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1600919226583

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 08 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Medical Rehabilitation Equipment Gait Training Robotic (ZEPU-AI1)?
The ZEPU-AI1 is a gait-training neuro-rehabilitation robot designed to assist stroke patients and others with mobility challenges to regain walking ability. It supports both upper- and lower-limb training and is intended for use in clinics, rehab centers, hospitals, and home settings.
2. What are the main features of this robot?
Key features include combined upper- and lower-limb training capability, service-free spare parts for reduced maintenance, CE and ISO certification for quality and safety, and a compact design suitable for varied clinical and home environments.
3. What certifications and instrument classification does the product have?
The device is classified as a Class I instrument and is CE and ISO certified.
4. What are the physical dimensions and space requirements?
The device dimensions are 2750 x 1100 x 2350 mm. Additional clearance may be required for patient access and therapist movement—please consult the manufacturer for recommended installation footprint and room layout.
5. Who is the device intended for and what clinical indications does it address?
The robot is intended for stroke patients and others with gait impairments. It is used for rehabilitation to improve walking skills and mobility, and for physical therapy in clinical and home-based programs.
6. Can the robot be used at home or only in clinical settings?
The robot is suitable for clinics, rehab centers, hospitals, and home use. For home installation, ensure appropriate space, supervision plans, and that a clinician has approved the device for the patient’s home program.
7. Is professional supervision required when using the device?
Yes. Rehabilitation robots should be used under the guidance of trained clinicians or therapists, particularly during initial sessions or for patients with significant deficits, to ensure safety and proper therapy progression.
8. What maintenance is required and what does 'service-free spare parts' mean?
The product is designed with service-free spare parts to minimize routine maintenance. Routine checks, cleaning, and following the manufacturer's maintenance guide are still recommended. For detailed maintenance schedules and procedures, contact the supplier.
9. How do I order, and what are the minimum order quantity (MOQ) and supply capacity?
The MOQ is 1 piece. The supplier's stated supply ability is 20 sets per month. To place an order or confirm availability, contact the manufacturer or authorized distributor.
10. Is OEM or customization available?
Yes. OEM options are available. Contact the manufacturer to discuss customization, branding, software or hardware options, and lead times for customized units.
11. What packaging and shipping details are provided?
Units are packaged in wooden boxes for dispatch. The stated port of dispatch is Qingdao. For shipping quotes, export documentation, or special packaging requests, contact the supplier.
12. What are the warranty and technical support options?
Warranty terms and technical support options are not specified in the product listing. Please contact the manufacturer or distributor for detailed warranty coverage, service contracts, training, and local support availability.
13. Are there any contraindications or safety precautions?
Specific contraindications are not listed in the product information. As with all rehabilitation equipment, use should be cleared by a treating physician and implemented by trained personnel. Patients with unstable medical conditions, severe osteoporosis, uncontrolled seizures, or other contraindicating conditions should not use the device until cleared by a clinician.
14. What patient data, software features, or therapy tracking does the device provide?
Software features and data-tracking capabilities are not detailed in the provided description. For information about therapy programs, data export, user interfaces, and electronic records, please request the product datasheet or software specification from the supplier.
15. Who should I contact for more technical specifications (power requirements, patient weight limits, installation)?
For full technical specifications such as electrical requirements, patient weight and size limits, installation instructions, and detailed clinical guidelines, contact the manufacturer or authorized distributor directly. They can provide the complete technical file and user manual.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading