B21, China Town Mall, Midrand
In-Situ Freeze Dryers Automatic Device Use Medicine Pharmacy Biological Research
- Section : Appliances
- Category : Food Processors
- SKU : 1601312345528

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 21 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the In-Situ Freeze Dryers Automatic Device?
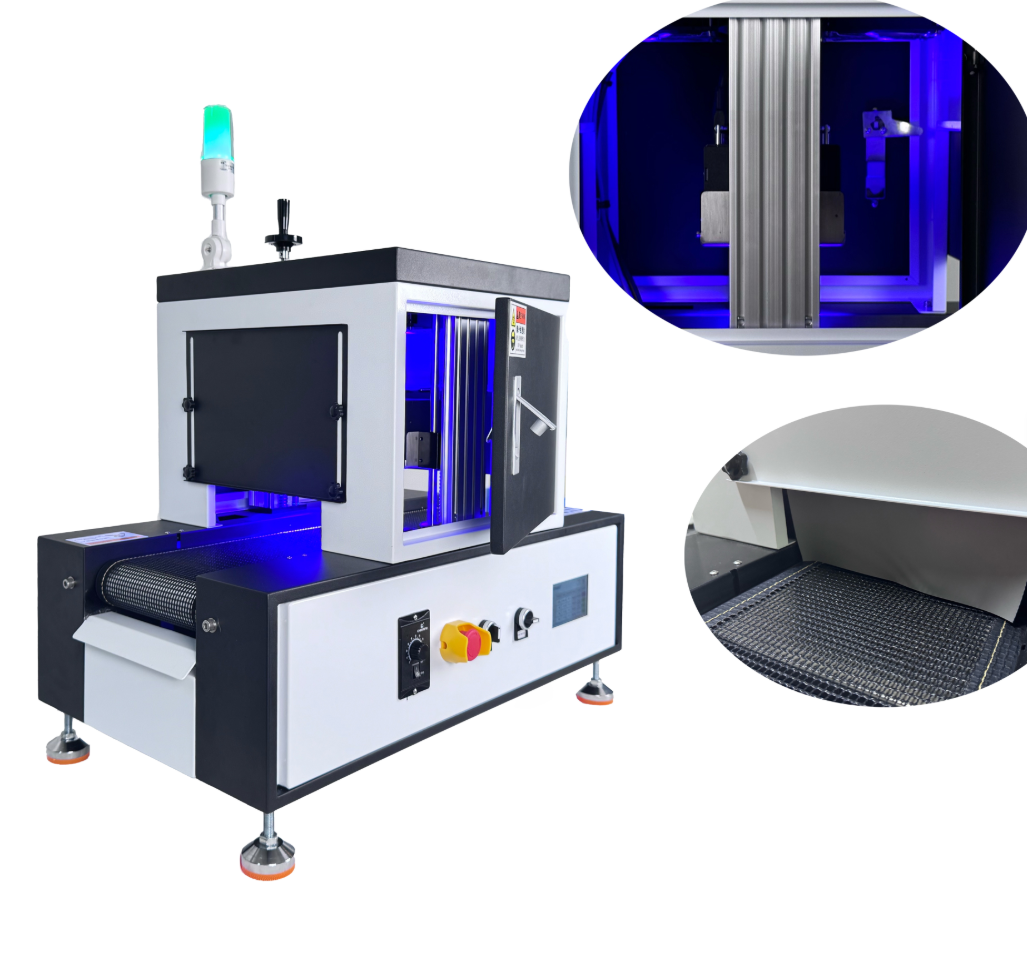
A benchtop automatic freeze-drying (lyophilization) device designed for pharmaceutical and biological research. It performs controlled freezing and sublimation to preserve sensitive materials and is offered in multiple models (HXLG-20FD, HXLG-20F, HXLG-20FT, HXLG-50F).
2. What applications is this device suitable for?
Typical uses include pharmaceutical R&D, lyophilized drug and reagent preparation, biological sample preservation (proteins, peptides, vaccines, tissues, cells), laboratory experiments requiring freeze-drying, and quality control in drug manufacturing.
3. What are the key certifications and quality assurances?
The device carries CE marking and ISO9001 certification, indicating compliance with applicable European safety requirements and a certified quality management system. For regulatory compliance in your region, check local requirements and supplier documentation.
4. What does 'Instrument Classification: Class IIIB' mean?
The product is listed as a Class IIIB instrument in the manufacturer's specifications, indicating its classification level for performance and intended use. Verify how this classification maps to your local medical/device regulatory categories before clinical or regulated use.
5. What power source does the device require?
The specification lists the power source as 'Other.' Exact electrical requirements depend on the selected model and regional configuration; confirm voltage, frequency, and plug type with the supplier or in the technical datasheet before purchase and installation.
6. How automatic is the operation and how do I control cycles?
The unit is described as automatic and user-friendly. It supports automated freeze-drying cycles (controlled freezing, primary and secondary drying). Specific control interfaces and programmable cycle features vary by model—refer to the model datasheet or contact support for UI details and training options.
7. What are the physical size and shipping weight?
Single package size is 56 x 45 x 45 cm and single gross weight is 12.000 kg. These indicate a compact benchtop footprint, but final installed footprint and clearances should be checked in the product manual.
8. How do I choose between the available models (HXLG-20FD / HXLG-20F / HXLG-20FT / HXLG-50F)?
Model choice depends on throughput needs, sample types, and any feature differences (e.g., tray configuration or temperature ranges). Contact the vendor or review detailed model datasheets for condenser capacity, shelf area, cycle automation features and any model-specific specs.
9. What types of samples are compatible with this freeze dryer?
Designed for a wide range of lab and pharmaceutical samples including aqueous solutions, biologicals (proteins, enzymes, vaccines), small tissue samples and formulations for lyophilization. Maximum sample volume and number of vials/shelves depend on the chosen model—refer to the model specifications.
10. What maintenance and cleaning are required?
Routine maintenance typically includes defrosting/cleaning the condenser, checking vacuum pump oil (if an external pump is used), inspecting seals and valves, and cleaning shelves and product-contact surfaces per manufacturer instructions. Follow the supplied maintenance schedule and keep records for regulated environments.
11. Is technical support available?
Yes—online technical support is available as stated in the product description. For on-site installation, training, spare parts or validation documentation, contact the supplier or authorized service representative.
12. What safety and biosafety precautions should I follow?
Follow standard laboratory safety: use appropriate PPE, handle infectious or hazardous materials in a suitable containment area (biosafety cabinet) before loading, ensure proper ventilation, and decontaminate the chamber and accessories as required. Consult the device manual and your institution's biosafety officer for specific procedures.
13. Can this device be used for regulated pharmaceutical production and what about validation?
The device is intended for pharmaceutical research and lab use. For regulated production, IQ/OQ/PQ and qualification documentation will be required. Contact the manufacturer for validation protocols, calibration certificates and documentation to support GMP or other regulatory requirements.
14. How do I order spare parts, consumables or request warranty information?
Contact the vendor or authorized distributor for spare parts, consumables (seals, valves, trays), and warranty terms. The manufacturer provides online technical support; they will supply part numbers, lead times and service/warranty details.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading