B21, China Town Mall, Midrand
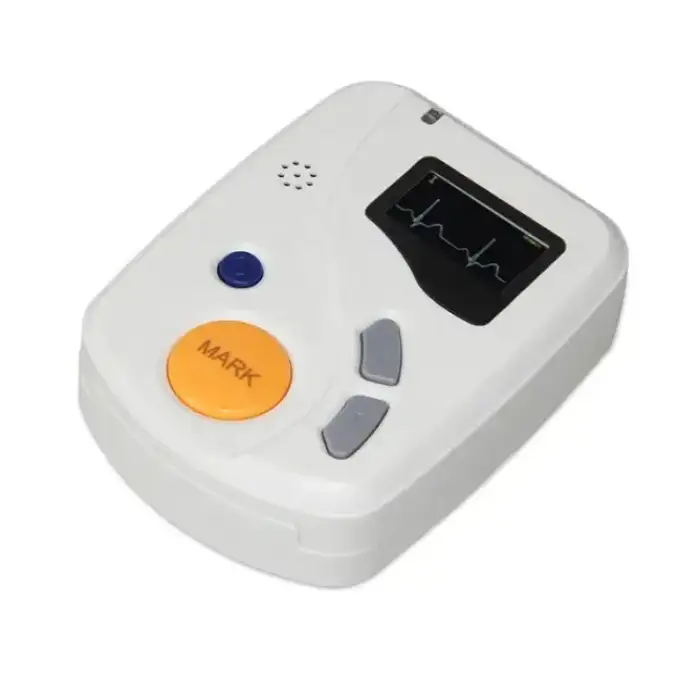
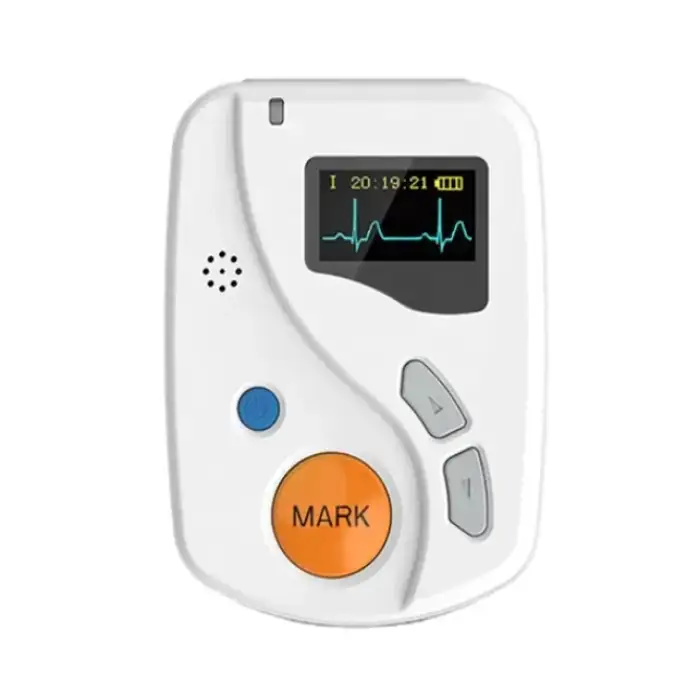
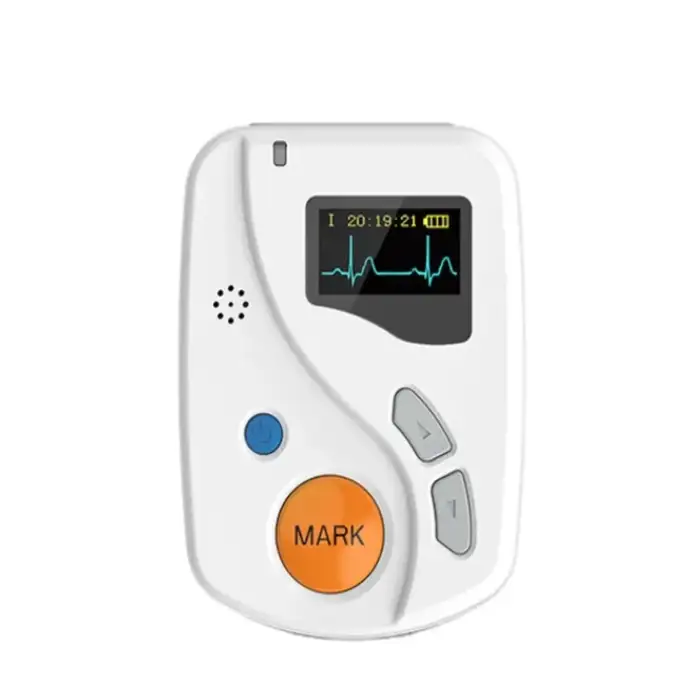
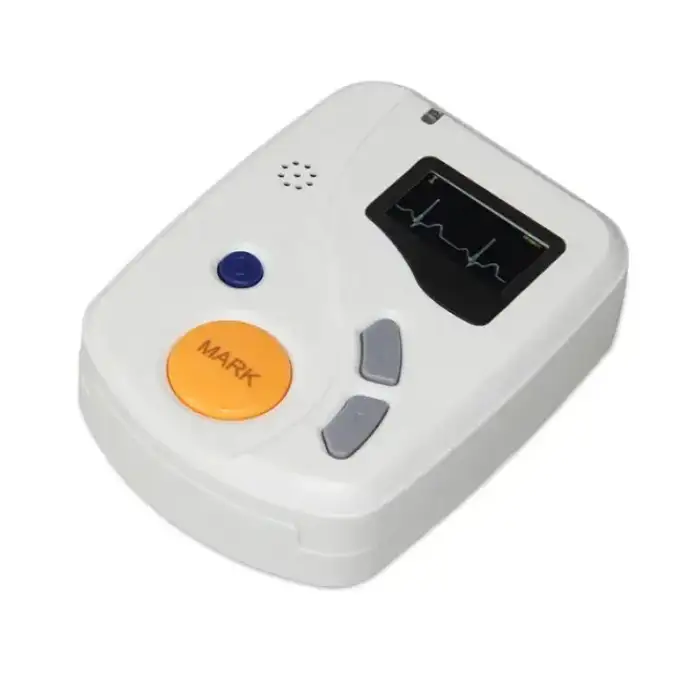
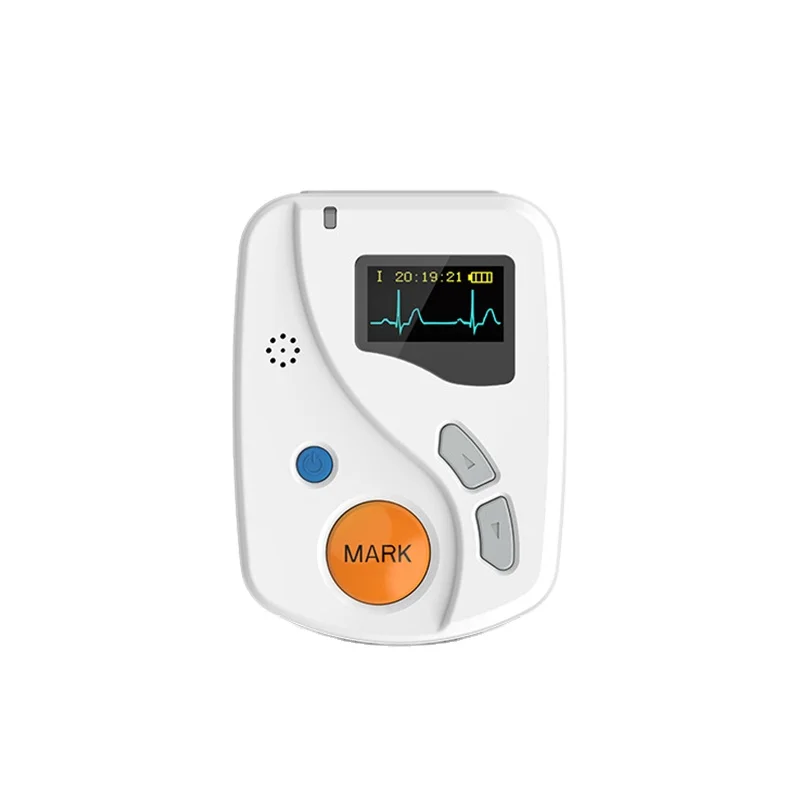
Good ECGH-K60 24 Hours Dynamic ECG System Medical Ambulatory Heart Holter Monitor ECG Machine
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1601167460814

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 20 Feb, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the main function of the ECGH-K60 monitor?
The ECGH-K60 monitor is designed for precise cardiac monitoring, providing accurate ECG readings in various medical settings, including hospitals and clinics.
2. How long can the ECGH-K60 monitor record ECG data?
The ECGH-K60 monitor can record ECG data continuously for up to 24 hours.
3. What type of battery does the ECGH-K60 use?
The ECGH-K60 uses one 'AAA' alkaline battery as its power source.
4. Is the ECGH-K60 portable?
Yes, the ECGH-K60 features a compact design and built-in battery, making it highly portable and suitable for remote monitoring.
5. What is the lead configuration of the ECGH-K60?
The ECGH-K60 utilizes a 12-lead standard lead configuration for comprehensive cardiac monitoring.
6. What is the timing accuracy of the ECGH-K60?
The overall timing error during a 24-hour recording shall not exceed 30 seconds.
7. What are the dimensions of the ECGH-K60 recorder?
The dimensions of the ECGH-K60 recorder are 81mm (L) x 60mm (W) x 22mm (H).
8. What kind of connectivity options does the ECGH-K60 have?
The ECGH-K60 features a USB 2.0 interface for easy data transfer and integration with other devices.
9. What is the input impedance of the ECGH-K60?
The input impedance of the ECGH-K60 is ≥10 MΩ.
10. What safety standards does the ECGH-K60 comply with?
The ECGH-K60 is classified as a Class II medical device but does not have a specified safety standard.
11. What is the common mode rejection (CMR) of the ECGH-K60?
The common mode rejection (CMR) of the ECGH-K60 is ≥60 dB.
12. How does the ECGH-K60 ensure the accuracy of readings?
The ECGH-K60 uses advanced technology to maintain low system noise (≤50 µVp-p) and minimal multichannel crosstalk (≤0.2 mV), ensuring accurate ECG readings.
13. Where is the ECGH-K60 manufactured?
The ECGH-K60 is manufactured in Jiangsu, China.
14. What certifications does the ECGH-K60 have?
The ECGH-K60 has an ISO quality certification, ensuring its reliability and quality in medical settings.
15. What is the minimum measurable signal for the ECGH-K60?
The minimum measurable signal for the ECGH-K60 is 50 µV.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading