B21, China Town Mall, Midrand
Generation Electrical Machinery Power Household Medical Device for Venous Thrombosis Prevention Blood
- Section : Appliances
- Category : Household Appliances
- SKU : 1601266691783

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 21 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
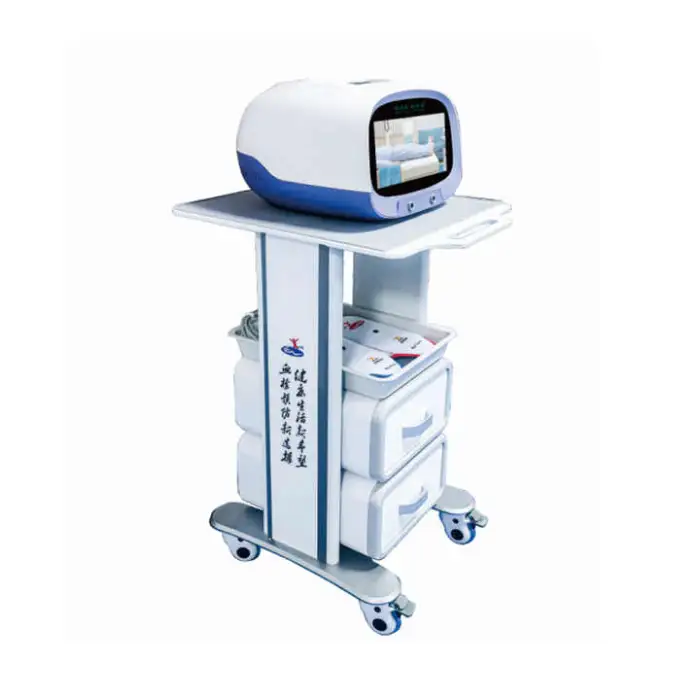
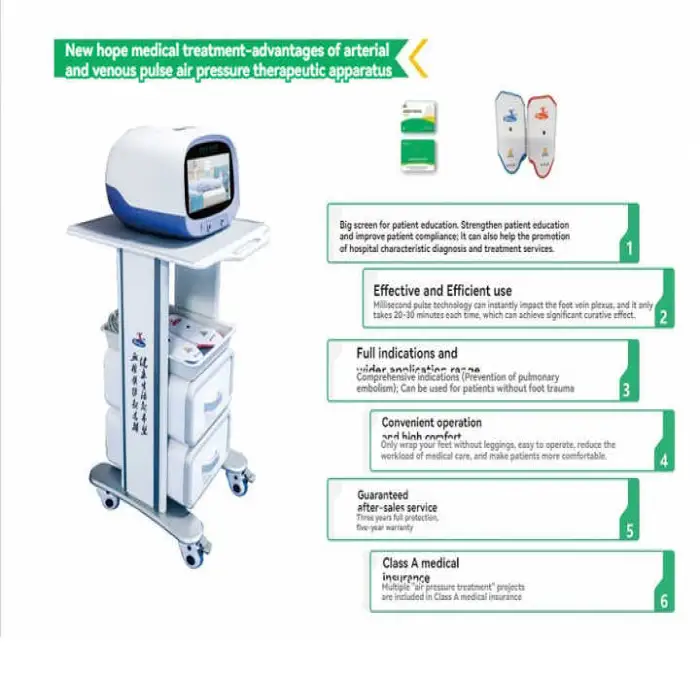
1. What is the Generation Electrical Machinery Power Household Medical Device for Venous Thrombosis Prevention Blood?
It is a Class II, CE‑certified, plug‑in household medical device designed to help promote blood circulation and reduce risk factors associated with venous thrombosis. It is intended for home healthcare applications, post‑surgery support, and long‑term management for at‑risk individuals.
2. Is this device medically certified and safe to use at home?
Yes — the device is classified as a Class II medical instrument and carries CE quality certification. It is designed for home use, but safety and proper use should be confirmed with your healthcare provider before starting therapy.
3. Who should use this device?
The device is intended for individuals at risk of venous thrombosis, those needing improved peripheral circulation, people recovering from surgery, and individuals with limited mobility. Always consult your physician to determine whether this device is appropriate for your condition.
4. Are there any contraindications or people who should not use it?
Patients with an active deep vein thrombosis (DVT), uncontrolled bleeding, certain skin conditions, or some implanted electronic medical devices (for example, pacemakers) should not use this device without medical clearance. Pregnant patients and those with other significant medical conditions should consult their healthcare provider first.
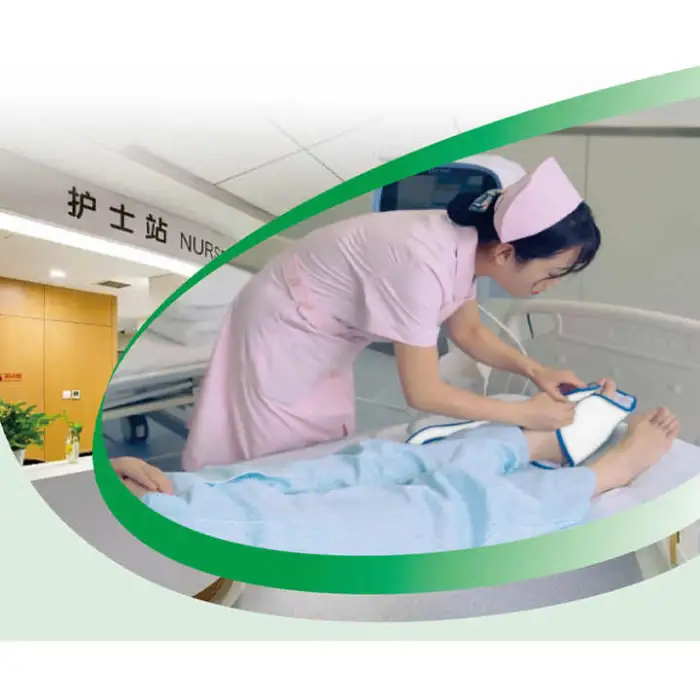
5. How do I use the device safely?
Read the user manual before first use, place the device or applicator as instructed, plug it into a proper electrical outlet, and follow any clinician‑provided therapy plan. Always inspect cords and connectors before use, keep the device dry, and unplug when not in use. If you experience pain, swelling, or unusual symptoms, stop use and contact your healthcare provider.
6. What is the recommended treatment duration and frequency?
Treatment duration and frequency vary by clinical need and should be determined by your healthcare provider. The device is intended to be used as directed by a clinician; follow their recommended protocol for session length and number of sessions per day.
7. What power source does the device require?
The device is powered by electricity and operates in a plug‑in power supply mode. It does not use internal batteries.
8. What are the product dimensions and weight?
The single package dimensions are 51.2 x 48.4 x 48.8 cm, and the single gross weight is approximately 5.2 kg.
9. What is the shelf life of the device?
The listed shelf life is 1 year. Check the product packaging and documentation for manufacturing or expiration dates and storage recommendations.
10. How do I clean and maintain the device?
Unplug the device before cleaning. Wipe external surfaces with a soft cloth dampened with mild disinfectant or soap solution; avoid soaking or introducing liquids into openings. Do not use abrasive cleaners or solvents. Regularly inspect cables and connectors for damage. Refer to the user manual for specific maintenance instructions.
11. Does the device come with technical support or a warranty?
Online technical support is available. Warranty coverage is not specified in the product summary—please check the seller's listing or contact the vendor directly for warranty terms, length, and support contact details.
12. What is included in the package and are accessories provided?
The product is sold as a single item. Included accessories and consumables (if any) may vary by seller or configuration—check the seller’s product listing or contact the vendor to confirm what is included.
13. Can this device replace prescribed anticoagulant medications?
No. This device is intended as a supportive therapy to promote circulation and reduce risk factors for venous thrombosis. It is not a substitute for prescribed anticoagulant medications or other clinician‑directed treatments. Always follow your physician’s treatment plan.
14. How should the device be stored when not in use?
Store the device in a clean, dry place away from direct sunlight, extreme temperatures, and humidity. Keep it out of reach of children. Follow any additional storage instructions provided in the user manual.
15. What should I do if the device malfunctions or causes discomfort?
Stop using the device immediately if you experience malfunction, pain, skin irritation, swelling, or other adverse effects. Contact the vendor’s technical support and your healthcare provider. Do not attempt to repair the device yourself unless instructed by authorized service personnel.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading