B21, China Town Mall, Midrand
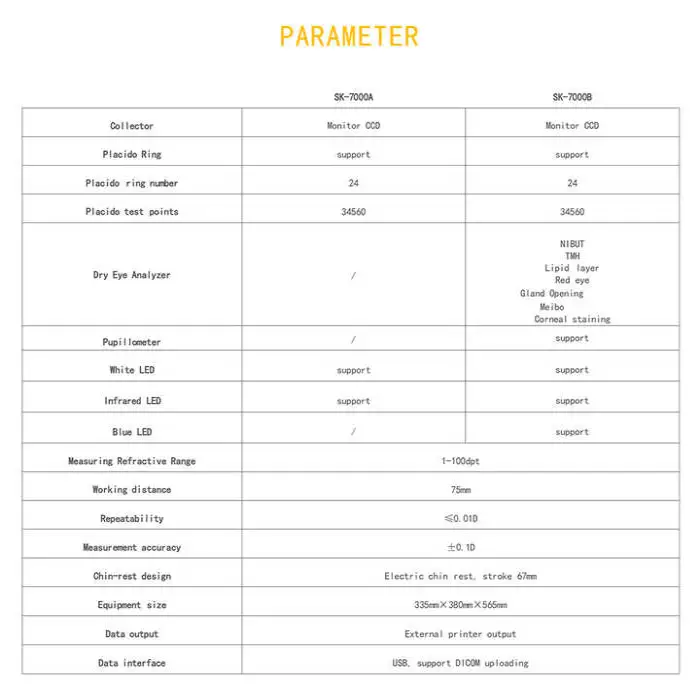
Corneal Topographer SK-7000B Ophthalmic Equipment Corneal Topography PLACIDO + DRY EYE + PUPILLOMETER
- Section : Consumer Electronics
- Category : Testing Equipment
- SKU : 1601396560630

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 02 Apr, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Corneal Topographer SK-7000B used for?
The SK-7000B is used for detailed corneal topography using Placido rings, assessment of dry eye and tear film, and precise pupil measurements. It supports pre-operative refractive surgery assessments, corneal disease monitoring, and ophthalmic research.
2. What measurement technology does the SK-7000B use?
The device uses a Placido-ring based imaging system (24 Placido rings) to capture corneal shape and curvature with 34,560 measured test points for high-resolution maps.
3. Does the SK-7000B perform dry eye evaluations?
Yes. The SK-7000B includes dry eye assessment features to evaluate tear film and ocular surface stability as part of routine ocular surface examinations.
4. Can the SK-7000B measure pupil size and dynamics?
Yes. The unit includes a pupilometer for accurate pupil diameter measurements and related pupillometry data used in diagnostics and pre-op planning.
5. What are the power and electrical requirements?
The SK-7000B is an electric device that supports a wide input voltage range of 110–220 V. Ensure your clinic power supply matches local electrical standards and use any recommended grounding or surge protection.
6. What are the physical dimensions and weight of the device?
The single package size is approximately 65 x 60 x 70 cm and the gross weight is around 25.0 kg. Allow additional space for installation, patient positioning, and operator access.
7. What connectivity and data export options are available?
The SK-7000B supports data export for reports and images. Typical options include USB and network connectivity; common output formats include image and numerical report files. For exact supported file types and EMR/DICOM compatibility, confirm with the supplier or product manual.
8. How long does a typical measurement take?
Captures are rapid—most topography and pupillometry measurements are obtained within seconds. Dry eye assessments may take additional time depending on the specific test protocol.
9. Is the SK-7000B suitable for use before refractive surgery?
Yes. Its high-resolution corneal maps and pupil measurements make it suitable for pre-operative evaluation and planning for refractive procedures, as well as postoperative monitoring.
10. What maintenance and cleaning are required?
Regular maintenance includes keeping optics and chin/forehead rests clean, performing software updates, and following calibration checks. Clean external surfaces with a soft cloth and manufacturer-approved disinfectant; never allow liquids to enter the instrument. Refer to the user manual for detailed procedures.
11. How often should the device be calibrated or serviced?
Calibration and servicing intervals depend on clinical usage and local regulations. Many clinics perform annual preventive maintenance and calibration, but follow the manufacturer’s recommendations and any local quality-control protocols.
12. Are there any patient contraindications or limitations?
Because the system relies on patient fixation and a clear tear film, limitations include poor fixation, severe eyelid/ocular surface disease, or active infections. The Placido method is non-contact and generally safe, but assess each patient individually.
13. What training is required to operate the SK-7000B?
Basic operator training is required to position patients, acquire high-quality captures, interpret output maps, and perform routine maintenance. Suppliers typically provide initial training and documentation; advanced clinical interpretation may require additional practitioner training.
14. What accessories are included or available?
Standard items typically include power and data cables, chin and forehead rests, and accompanying software. Optional accessories may include printers, extended warranty packages, and network integration kits. Check with the vendor for the exact package contents.
15. What warranty and technical support options are available?
Warranty and support vary by supplier and region. Most vendors offer a standard warranty plus optional extended service contracts, remote software updates, and on-site support. Contact the authorized dealer for specific warranty terms and support plans.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading